Chapter 5: Introductory Atomic Theory and Structure
Enhanced Introductory College Chemistry
by Gregory Anderson; Caryn Fahey; Jackie MacDonald; Adrienne Richards; Samantha Sullivan Sauer; J.R. van Haarlem; and David Wegman;
Chapter Contents
- 5.1 Early Atomic Theory: Dalton’s Model of the Atom
- 5.2 Electric Charge
- 5.3 Subatomic Particles of the Atom
- 5.4 Defining the Nuclear Atom
- 5.5 Isotopes of the Elements
- 5.6 Atomic Mass
- Summary
- Review
Except where otherwise noted, this OER is licensed under CC BY 4.0
Please visit the web version of Enhanced Introductory College Chemistry to access the complete book, interactive activities and ancillary resources.
In this chapter, you will learn about
- Dalton’s atomic theory
- Basic concepts in electric charge
- The discoveries of an atom’s subatomic particles atom including electrons, protons, and neutrons
- The structure of the nuclear atom and how neutral atoms form ions
- Isotopes of elements and how to read and write isotope symbols
- Atomic masses of elements and their isotopes and how to calculate percent abundance.
To better support your learning, you should be familiar with the following concepts before starting this chapter:
- How to reference element names, symbols, and masses from the periodic table
- How to use your scientific calculator to perform percent calculations
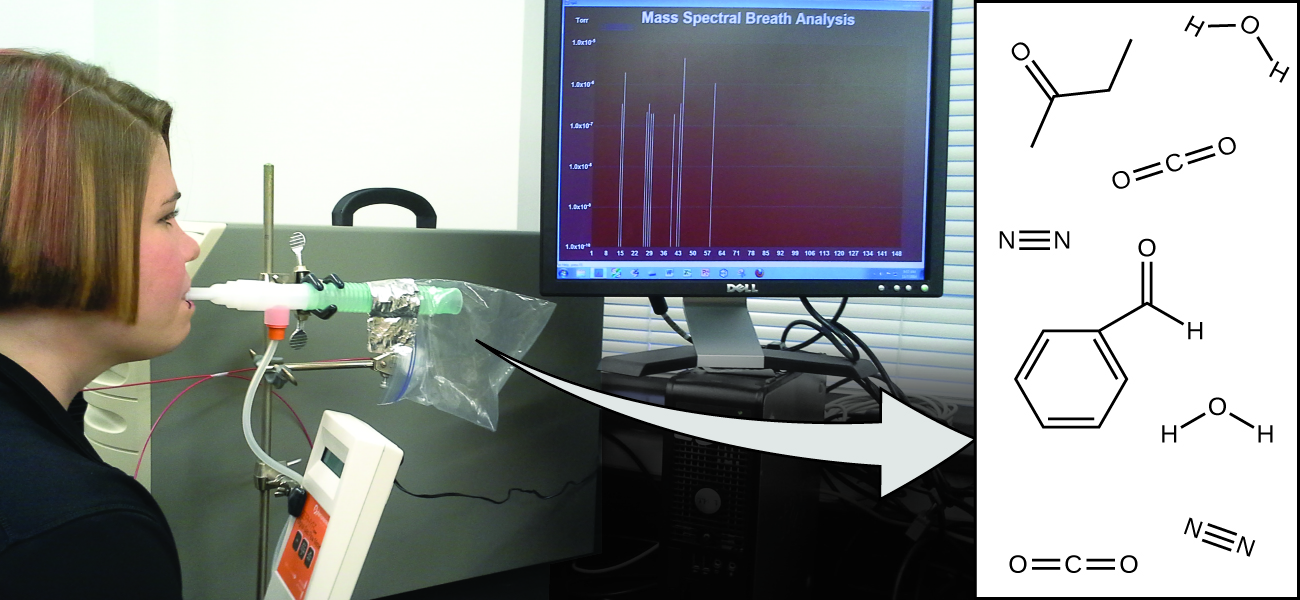
Your overall health and susceptibility to disease depends upon the complex interaction between your genetic makeup and environmental exposure, with the outcome difficult to predict. Early detection of biomarkers, substances that indicate an organism’s disease or physiological state, could allow diagnosis and treatment before a condition becomes serious or irreversible. Recent studies have shown that your exhaled breath can contain molecules that may be biomarkers for recent exposure to environmental contaminants or for pathological conditions ranging from asthma to lung cancer (Figure 5a). Scientists are working to develop biomarker “fingerprints” that could be used to diagnose a specific disease based on the amounts and identities of certain molecules in a patient’s exhaled breath. An essential concept underlying this goal is that of a molecule’s identity, which is determined by the numbers and types of atoms it contains, and how they are bonded together. This chapter will describe some of the fundamental chemical principles related to the composition of matter, including those central to the concept of molecular identity.