Chapter 9 – Review
9.1 Stoichiometry Basics; and 9.2 Mole-Mass and Mass-Mass Calculations
- Write the balanced equation, then outline the steps necessary to determine the information requested in each of the following:
- The number of moles and the mass of chlorine, Cl2, required to react with 10.0 g of sodium metal, Na, to produce sodium chloride, NaCl.
- The number of moles and the mass of oxygen formed by the decomposition of 1.252 g of mercury(II) oxide.
- The number of moles and the mass of sodium nitrate, NaNO3, required to produce 128 g of oxygen. (NaNO2 is the other product.)
- The number of moles and the mass of carbon dioxide formed by the combustion of 20.0 kg of carbon in an excess of oxygen.
- The number of moles and the mass of copper(II) carbonate needed to produce 1.500 kg of copper(II) oxide. (CO2 is the other product.)
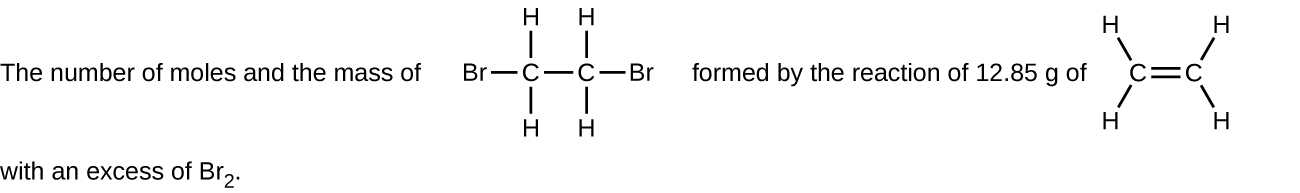
- Determine the number of moles and the mass requested for each reaction in Q.1 a) to f) above. Check Answer: [1]
- Write the balanced equation, then outline the steps necessary to determine the information requested in each of the following
- The number of moles and the mass of Mg required to react with 5.00 g of HCl and produce MgCl2 and H2.
- The number of moles and the mass of oxygen formed by the decomposition of 1.252 g of silver(I) oxide.
- The number of moles and the mass of magnesium carbonate, MgCO3, required to produce 283 g of carbon dioxide. (MgO is the other product.)
- The number of moles and the mass of water formed by the combustion of 20.0 kg of acetylene, C2H2, in an excess of oxygen.
- The number of moles and the mass of barium peroxide, BaO2, needed to produce 2.500 kg of barium oxide, BaO (O2 is the other product.)

- Determine the number of moles and the mass requested for each reaction in Q3. a) to f) above. Check Answer: [2]
- I2 is produced by the reaction of 0.4235 mol of CuCl2 according to the following equation: [latex]2\text{CuCl}_2 + 4\text{KI} \longrightarrow 2\text{CuI} + 4\text{KCl} + \text{I}_2[/latex].
(a) What mass of I2 is produced?
- Silver is often extracted from ores such as K[Ag(CN)2] and then recovered by the reaction
[latex]2 \text{K} [\text{Ag(CN)}_2](aq) + \text{Zn}(s) \longrightarrow 2\text{Ag}(s) + \text{Zn(CN)}_2(aq) + 2\text{KCN}(aq)[/latex](a) What mass of Zn(CN)2 is produced? Check Answer: [3] -
What mass of CO2 is produced by the combustion of 1.00 mol of CH4? Check Answer: [4]
CH4(g) + 2O2(g) → CO2(g) + 2H2O(ℓ)
-
What mass of H2O is produced by the combustion of 1.00 mol of CH4?
CH4(g) + 2O2(g) → CO2(g) + 2H2O(ℓ)
-
What mass of HgO is required to produce 0.692 mol of O2? Check Answer: [5]
2HgO(s) → 2Hg(ℓ) + O2(g)
-
What mass of NaHCO3 is needed to produce 2.659 mol of CO2?
2NaHCO3(s) → Na2CO3(s) + H2O(ℓ) + CO2(g)
-
How many moles of Al can be produced from 10.87 g of Ag? Check Answer: [6]
Al(NO3)3(s) + 3Ag → Al + 3AgNO3
-
How many moles of HCl can be produced from 0.226 g of SOCl2?
SOCl2(ℓ) + H2O(ℓ) → SO2(g) + 2HCl(g)
-
How many moles of O2 are needed to prepare 1.00 g of Ca(NO3)2? Check Answer: [7]
Ca(s) + N2(g) + 3O2(g) → Ca(NO3)2(s)
-
How many moles of C2H5OH are needed to generate 106.7 g of H2O?
C2H5OH(ℓ) + 3O2(g) → 2CO2(g) + 3H2O(ℓ)
-
What mass of O2 can be generated by the decomposition of 100.0 g of NaClO3? Check Answer: [8]
2NaClO3 → 2NaCl(s) + 3O2(g)
-
What mass of Li2O is needed to react with 1,060 g of CO2?
Li2O(aq) + CO2(g) → Li2CO3(aq)
-
What mass of Fe2O3 must be reacted to generate 324 g of Al2O3? Check Answer: [9]
Fe2O3(s) + 2Al(s) → 2Fe(s) + Al2O3(s)
-
What mass of Fe is generated when 100.0 g of Al are reacted?
Fe2O3(s) + 2Al(s) → 2Fe(s) + Al2O3(s)
-
What mass of MnO2 is produced when 445 g of H2O are reacted? Check Answer: [10]
H2O(ℓ) + 2MnO4−(aq) + Br−(aq) → BrO3−(aq) + 2MnO2(s) + 2OH−(aq)
-
What mass of PbSO4 is produced when 29.6 g of H2SO4 are reacted?
Pb(s) + PbO2(s) + 2H2SO4(aq) → 2PbSO4(s) + 2H2O(ℓ)
-
If 83.9 g of ZnO are formed, what mass of Mn2O3 is formed with it? Check Answer: [11]
Zn(s) + 2MnO2(s) → ZnO(s) + Mn2O3(s)
-
If 14.7 g of NO2 are reacted, what mass of H2O is reacted with it?
3NO2(g) + H2O(ℓ) → 2HNO3(aq) + NO(g)
-
If 88.4 g of CH2S are reacted, what mass of HF is produced? Check Answer: [12]
CH2S + 6F2 → CF4 + 2HF + SF6
-
If 100.0 g of Cl2 are needed, what mass of NaOCl must be reacted?
NaOCl + HCl → NaOH + Cl2
- What mass of silver oxide, Ag2O, is required to produce 25.0 g of silver sulfadiazine, AgC10H9N4SO2, from the reaction of silver oxide and sulfadiazine?
[latex]2\text{C}_{10} \text{H}_{10} \text{N}_4 \text{SO}_2 + \text{Ag}_2 \text{O} \longrightarrow 2\text{Ag} \text{C}_{10} \text{H}_{9} \text{N}_4 \text{SO}_2 + \text{H}_2 \text{O}[/latex] - Carborundum is silicon carbide, SiC, a very hard material used as an abrasive on sandpaper and in other applications. It is prepared by the reaction of pure sand, SiO2, with carbon at high temperature. Carbon monoxide, CO, is the other product of this reaction. Write the balanced equation for the reaction, and calculate how much SiO2 is required to produce 3.00 kg of SiC. Check Answer: [13]
9.3 Limiting Reactants and 9.4 Reaction Yields
- Urea, CO(NH2)2, is manufactured on a large scale for use in producing urea-formaldehyde plastics and as a fertilizer. What is the maximum mass of urea that can be manufactured from the CO2 produced by combustion of 1.00×103kg of carbon followed by the reaction? Check Answer: [14]
[latex]\text{CO}_2(g) + 2\text{NH}_3(g) \longrightarrow {\text{CO(NH}_2})_2(s) + \text{H}_2 \text{O}(l)[/latex] - In an accident, a solution containing 2.5 kg of nitric acid was spilled. Two kilograms of Na2CO3 was quickly spread on the area and CO2 was released by the reaction. Was sufficient Na2CO3 used to neutralize all of the acid?
- A compact car gets 37.5 miles per gallon on the highway. If gasoline contains 84.2% carbon by mass and has a density of 0.8205 g/mL, determine the mass of carbon dioxide produced during a 500-mile trip (3.785 litres per gallon).
- What is the limiting reactant in a reaction that produces sodium chloride from 8 g of sodium and 8 g of diatomic chlorine? Check Answer: [15]
- Which of the postulates of Dalton’s atomic theory explains why we can calculate a theoretical yield for a chemical reaction?
- A student isolated 25 g of a compound following a procedure that would theoretically yield 81 g. What was his percent yield? Check Answer: [16]
- A sample of 0.53 g of carbon dioxide was obtained by heating 1.31 g of calcium carbonate. What is the percent yield for this reaction?
[latex]\text{CaCO}_3(s) \longrightarrow \text{CaO}(s) + \text{CO}_2(s)[/latex] - Freon-12, CCl2F2, is prepared from CCl4 by reaction with HF. The other product of this reaction is HCl. Outline the steps needed to determine the percent yield of a reaction that produces 12.5 g of CCl2F2 from 32.9 g of CCl4. Freon-12 has been banned and is no longer used as a refrigerant because it catalyzes the decomposition of ozone and has a very long lifetime in the atmosphere. Determine the percent yield. Check Answer: [17]
- Citric acid, C6H8O7, a component of jams, jellies, and fruity soft drinks, is prepared industrially via fermentation of sucrose by the mold Aspergillus niger. The equation representing this reaction is
[latex]\text{C}_{12}\text{H}_{22}\text{O}_{11}\;+\;\text{H}_2\text{O}\;+3\text{O}_2\;{\longrightarrow}\;2\text{C}_6\text{H}_8\text{O}_7\;+\;4\text{H}_2\text{O}[/latex]
What mass of citric acid is produced from exactly 1 metric ton (1.000 × 103 kg) of sucrose if the yield is 92.30%? - Toluene, C6H5CH3, is oxidized by air under carefully controlled conditions to benzoic acid, C6H5CO2H, which is used to prepare the food preservative sodium benzoate, C6H5CO2Na. What is the percent yield of a reaction that converts 1.000 kg of toluene to 1.21 kg of benzoic acid? Check Answer: [18]
[latex]2\text{C}_6\text{H}_5\text{CH}_3\;+\;3\text{O}_2\;{\longrightarrow}\;2\text{C}_6\text{H}_5\text{CO}_2\text{H}\;+\;2\text{H}_2\text{O}[/latex] - In a laboratory experiment, the reaction of 3.0 mol of H2 with 2.0 mol of I2 produced 1.0 mol of HI. Determine the theoretical yield in grams and the percent yield for this reaction.
- Outline the steps needed to determine the limiting reactant when 30.0 g of propane, C3H8, is burned with 75.0 g of oxygen. Determine the limiting reactant.
- Outline the steps needed to determine the limiting reactant when 0.50 mol of Cr and 0.75 mol of H3PO4 react according to the following chemical equation.
[latex]2\text{Cr}\;+\;2\text{H}_3\text{PO}_4\;{\longrightarrow}\;2\text{CrPO}_4\;+\;3\text{H}_2[/latex]
Determine the limiting reactant. Check Answer: [19] - What is the limiting reactant when 1.50 g of lithium and 1.50 g of nitrogen combine to form lithium nitride, a component of advanced batteries, according to the following unbalanced equation?
- Uranium can be isolated from its ores by dissolving it as UO2(NO3)2, then separating it as solid UO2(C2O4)·3H2O. Addition of 0.4031 g of sodium oxalate, Na2C2O4, to a solution containing 1.481 g of uranyl nitrate, UO2(NO3)2, yields 1.073 g of solid UO2(C2O4)·3H2O.
[latex]\text{Na}_2\text{C}_2\text{O}_4\;+\;\text{UO}_2(\text{NO}_3)_2\;+\;3\text{H}_2\text{O}\;{\longrightarrow}\;\text{UO}_2(\text{C}_2\text{O}_4){\cdot}3\text{H}_2\text{O}\;+\;2\text{NaNO}_3[/latex]
Determine the limiting reactant and the percent yield of this reaction. Check Answer: [20] - The phosphorus pentoxide used to produce phosphoric acid for cola soft drinks is prepared by burning phosphorus in oxygen.
- What is the limiting reactant when 0.200 mol of P4 and 0.200 mol of O2 react according to [latex]\text{P}_4\;+\;5\text{O}_2\;{\longrightarrow}\;\text{P}_4\text{O}_{10}[/latex]
- Calculate the percent yield if 10.0 g of P4O10 is isolated from the reaction.
Attribution & References
Except where otherwise noted, this page is adapted by Adrienne Richards from:
- “Ch. 4 Exercises” In Chemistry 2e (OpenStax) by Paul Flowers, Klaus Theopold, Richard Langley, & William R. Robinson, licensed under CC BY 4.0. Access for free at Chemistry 2e (OpenStax). / Adapted exercises 4.3 and 4.4 for this page.
- “Chapter 5: Stoichiometry and The Mole: Mole-Mass and Mass-Mass Calculations“ In Introductory Chemistry: 1st Canadian Edition by David W. Ball and Jessica A. Key, licensed under CC BY-NC-SA 4.0. / Adapted exercises 7-24 for this page.
- (a) 0.435 mol Na, 0.217 mol Cl2, 15.4 g Cl2; (b) 0.005780 mol HgO, 2.890 × 10−3 mol O2, 9.248 × 10−2 g O2; (c) 8.00 mol NaNO3, 6.8 × 102 g NaNO3; (d) 1665 mol CO2, 73.3 kg CO2; (e) 18.86 mol CuO, 2.330 kg CuCO3; (f) 0.4580 mol C2H4Br2, 86.05 g C2H4Br2 ↵
- (a) 0.0686 mol Mg, 1.67 g Mg; (b) 2.701 × 10−3 mol O2, 0.08644 g O2; (c) 6.43 mol MgCO3, 542 g MgCO3 (d) 713 mol H2O, 12.8 kg H2O; (e) 16.31 mol BaO2, 2762 g BaO2; (f) 0.207 mol C2H4, 5.81 g C2H4 ↵
- (a) 10.41 g Zn(CN)2 ↵
- 44.0 g ↵
- 3.00 × 102 g ↵
- 0.0336 mol ↵
- 0.0183 mol ↵
- 45.1 g ↵
- 507 g ↵
- 4.30 × 103 g ↵
- 163 g ↵
- 76.7 g ↵
- [latex]\text{SiO}_2 + 3\text{C} \longrightarrow \text{SiC} + 2\text{CO}[/latex], 4.50 kg SiO2 ↵
- 5.00 × 103 kg ↵
- The limiting reactant is Cl2. ↵
- Percent yield = 31% ↵
- g CCl4 → mol CCl4 → mol CCl2F2 → g CCl2F2, percent yield = 48.3% ↵
- percent yield = 91.3% ↵
- The conversion needed is mol Cr→ mol H3PO4. Then compare the amount of Cr to the amount of acid present. Cr is the limiting reactant. ↵
- Na2C2O4 is the limiting reactant. percent yield = 86.6% ↵

