Chapter 7 – Review
7.1 The Mole Concept and Avogadro’s Number; and 7.2 Molecular Mass, Avogadro’s Number and The Mole
- What is the total mass (amu and g/mol) of carbon in each of the following molecules?
- CH4
- CHCl3
- C12H10O6
- CH3CH2CH2CH2CH3
Check Answer: [1]
- What is the total mass (amu and g/mol) of hydrogen in each of the molecules?
- CH4
- CHCl3
- C12H10O6
- CH3CH2CH2CH2CH3
- Calculate the molecular or formula mass and molar mass of each of the following:
- P4
- H2O
- Ca(NO3)2
- CH3CO2H (acetic acid)
- C12H22O11 (sucrose, cane sugar).
Check Answer: [2]
- Determine the molecular mass and molar mass of the following compounds:
(a)
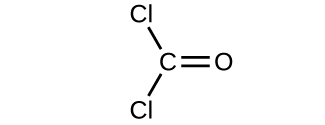
(b)
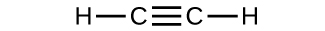
(c)
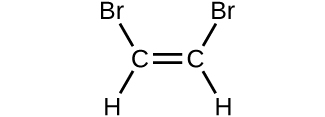
(d)
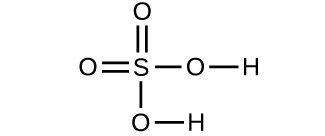
- Determine the molecular mass and molar mass of the following compounds: Check Answer: [3]
(a)
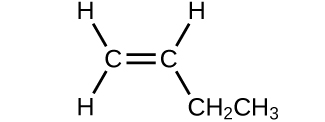
(b)
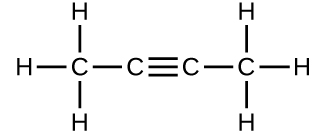
(c)
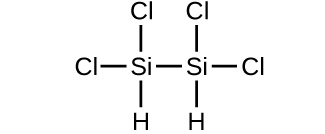
(d)
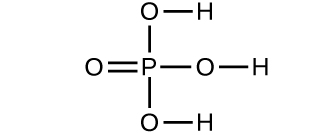
- Which molecule has a molecular mass of 28.05 amu?
(a)
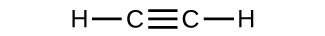
(b)
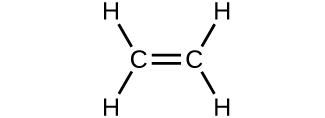
(c)
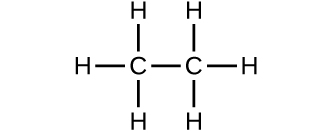
- Write a sentence that describes how to determine the number of moles of a compound in a known mass of the compound if we know its molecular formula. Check Answer: [4]
- Compare 1 mole of H2, 1 mole of O2, and 1 mole of F2.
- Which has the largest number of molecules? Explain why.
- Which has the greatest mass? Explain why.
- Which contains the greatest mass of oxygen: 0.75 mol of ethanol (C2H5OH), 0.60 mol of formic acid (HCO2H), or 1.0 mol of water (H2O)? Explain why. Check Answer: [5]
- Which contains the greatest number of moles of oxygen atoms: 1 mol of ethanol (C2H5OH), 1 mol of formic acid (HCO2H), or 1 mol of water (H2O)? Explain why.
- How are the molecular mass and the molar mass of a compound similar and how are they different? Check Answer: [6]
- Calculate the molar mass of each of the following compounds:
- hydrogen fluoride, HF
- ammonia, NH3
- nitric acid, HNO3
- silver sulfate, Ag2SO4
- boric acid, B(OH)3
- Calculate the molar mass of each of the following:
- S8
- C5H12
- Sc2(SO4)3
- CH3COCH3 (acetone)
- C6H12O6 (glucose)
Check Answer: [7]
- Calculate the empirical or molecular formula mass and the molar mass of each of the following minerals:
- limestone, CaCO3
- halite, NaCl
- beryl, Be3Al2Si6O18
- malachite, Cu2(OH)2CO3
- turquoise, CuAl6(PO4)4(OH)8(H2O)4
- Calculate the molar mass of each of the following:
- the anesthetic halothane, C2HBrClF3
- the herbicide paraquat, C12H14N2Cl2
- caffeine, C8H10N4O2
- urea, CO(NH2)2
- a typical soap, C17H35CO2Na
Check Answer: [8]
- Determine the number of moles of compound and the number of moles of each type of atom in each of the following:
- 25.0 g of propylene, C3H6
- 3.06 × 10−3 g of the amino acid glycine, C2H5NO2
- 25 lb of the herbicide Treflan, C13H16N2O4F (1 lb = 454 g)
- 0.125 kg of the insecticide Paris Green, Cu4(AsO3)2(CH3CO2)2
- 325 mg of aspirin, C6H4(CO2H)(CO2CH3)
- Determine the mass of each of the following:
- 0.0146 mol KOH
- 10.2 mol ethane, C2H6
- 1.6 × 10−3 mol Na2 SO4
- 6.854 × 103 mol glucose, C6 H12 O6
- 2.86 mol Co(NH3)6Cl3
Check Answer: [9]
- Determine the number of moles of the compound and determine the number of moles of each type of atom in each of the following:
- 2.12 g of potassium bromide, KBr
- 0.1488 g of phosphoric acid, H3PO4
- 23 kg of calcium carbonate, CaCO3
- 78.452 g of aluminum sulfate, Al2(SO4)3
- 0.1250 mg of caffeine, C8H10N4O2
- Determine the mass of each of the following:
- 2.345 mol LiCl
- 0.0872 mol acetylene, C2H2
- 3.3 × 10−2 mol Na2 CO3
- 1.23 × 103 mol fructose, C6 H12 O6
- 0.5758 mol FeSO4(H2O)7
Check Answer: [10]
- The approximate minimum daily dietary requirement of the amino acid leucine, C6H13NO2, is 1.1 g. What is this requirement in moles?
- Determine the mass in grams of each of the following:
- 0.600 mol of oxygen atoms
- 0.600 mol of oxygen molecules, O2
- 0.600 mol of ozone molecules, O3
Check Answer: [11]
- A 55-kg woman has 7.5 × 10−3 mol of hemoglobin (molar mass = 64,456 g/mol) in her blood. How many hemoglobin molecules is this? What is this quantity in grams?
- Determine the number of atoms and the mass of zirconium, silicon, and oxygen found in 0.3384 mol of zircon, ZrSiO4, a semiprecious stone. Check Answer: [12]
- Determine which of the following contains the greatest mass of hydrogen: 1 mol of CH4, 0.6 mol of C6H6, or 0.4 mol of C3H8.
- Determine which of the following contains the greatest mass of aluminum: 122 g of AlPO4, 266 g of Al2C16, or 225 g of Al2S3. Check Answer: [13]
- Diamond is one form of elemental carbon. An engagement ring contains a diamond weighing 1.25 carats (1 carat = 200 mg). How many atoms are present in the diamond?
- The Cullinan diamond was the largest natural diamond ever found (January 25, 1905). It weighed 3104 carats (1 carat = 200 mg). How many carbon atoms were present in the stone? Check Answer: [14]
- One 55-gram serving of a particular cereal supplies 270 mg of sodium, 11% of the recommended daily allowance. How many moles and atoms of sodium are in the recommended daily allowance?
- A certain nut crunch cereal contains 11.0 grams of sugar (sucrose, C12H22O11) per serving size of 60.0 grams. How many servings of this cereal must be eaten to consume 0.0278 moles of sugar? Check Answer: [15]
- A tube of toothpaste contains 0.76 g of sodium monofluorophosphate (Na2PO3F) in 100 mL.
- What mass of fluorine atoms in mg was present?
- How many fluorine atoms were present?
- Which of the following represents the least number of molecules?
- 20.0 g of H2O (18.02 g/mol)
- 77.0 g of CH4 (16.06 g/mol)
- 68.0 g of CaH2 (42.09 g/mol)
- 100.0 g of N2O (44.02 g/mol)
- 84.0 g of HF (20.01 g/mol)
Check Answer: [16]
7.3 Percent Composition; and 7.4 Determining Empirical and Molecular Formulas
- What information do we need to determine the molecular formula of a compound from the empirical formula?
- Calculate the following to four significant figures:
- the percent composition of ammonia, NH3
- the percent composition of photographic “hypo,” Na2S2O3
- the percent of calcium ion in Ca3(PO4)2
Check Answer: [17]
- Determine the following to four significant figures:
- the percent composition of hydrazoic acid, HN3
- the percent composition of TNT, C6H2(CH3)(NO2)3
- the percent of SO42– in Al2(SO4)3
- Determine the percent ammonia, NH3, in Co(NH3)6Cl3, to three significant figures. Check Answer: [18]
- Determine the percent water in CuSO4∙5H2O to three significant figures.
- Determine the empirical formulas for compounds with the following percent compositions:
- 15.8% carbon and 84.2% sulfur
- 40.0% carbon, 6.7% hydrogen, and 53.3% oxygen
Check Answer: [19]
- Determine the empirical formulas for compounds with the following percent compositions:
- 43.6% phosphorus and 56.4% oxygen
- 28.7% K, 1.5% H, 22.8% P, and 47.0% O
- A compound of carbon and hydrogen contains 92.3% C and has a molar mass of 78.1 g/mol. What is its molecular formula? Check Answer: [20]
- Dichloroethane, a compound that is often used for dry cleaning, contains carbon, hydrogen, and chlorine. It has a molar mass of 99 g/mol. Analysis of a sample shows that it contains 24.3% carbon and 4.1% hydrogen. What is its molecular formula?
- Determine the empirical and molecular formula for chrysotile asbestos. Chrysotile has the following percent composition: 28.03% Mg, 21.60% Si, 1.16% H, and 49.21% O. The molar mass for chrysotile is 520.8 g/mol. Check Answer: [21]
- Polymers are large molecules composed of simple units repeated many times. Thus, they often have relatively simple empirical formulas. Calculate the empirical formulas of the following polymers:
- Lucite (Plexiglas); 59.9% C, 8.06% H, 32.0% O
- Saran; 24.8% C, 2.0% H, 73.1% Cl
- polyethylene; 86% C, 14% H
- polystyrene; 92.3% C, 7.7% H
- Orlon; 67.9% C, 5.70% H, 26.4% N
- A major textile dye manufacturer developed a new yellow dye. The dye has a percent composition of 75.95% C, 17.72% N, and 6.33% H by mass with a molar mass of about 240 g/mol. Determine the molecular formula of the dye. Check Answer: [22]
Attribution & References
Except where otherwise noted, this section is adapted from “Ch. 3 Exercises 3.1 & 3.2” In Chemistry 2e (OpenStax) by Paul Flowers, Klaus Theopold, Richard Langley, & William R. Robinson, licensed under CC BY 4.0. / Adaptations include extracting the exercises relevant to this chapter from 3.1, 3.2.
- (a) 12.01 amu or g/mol; (b) 12.01 amu or g/mol; (c) 144.12 amu or g/mol; (d) 60.05 amu or g/mol ↵
- (a) 123.896 amu or g/mol; (b) 18.015 amu or g/mol; (c) 164.086 amu or g/mol; (d) 60.052 amu or g/mol; (e) 342.297 amu or g/mol ↵
- (a) 56.107 amu or g/mol; (b) 54.091 amu or g/mol; (c) 199.9976 amu or g/mol; (d) 97.9950 amu or g/mol ↵
- Use the molecular formula to find the molar mass; to obtain the number of moles, divide the mass of compound by the molar mass of the compound expressed in grams. ↵
- Formic acid. Its formula has twice as many oxygen atoms as the other two compounds (one each). Therefore, 0.60 mol of formic acid would be equivalent to 1.20 mol of a compound containing a single oxygen atom. ↵
- The two masses have the same numerical value, but the units are different: The molecular mass is the mass of 1 molecule while the molar mass is the mass of 6.022 × 1023 molecules. ↵
- (a) 256.528 g/mol; (b) 72.150 g mol−1; (c) 378.103 g mol−1; (d) 58.080 g mol−1; (e) 180.158 g mol−1 ↵
- (a) 197.382 g mol−1; (b) 257.163 g mol−1; (c) 194.193 g mol−1; (d) 60.056 g mol−1; (e) 306.464 g mol−1 ↵
- (a) 0.819 g; (b) 307 g; (c) 0.23 g; (d) 1.235 × 106 g (1235 kg); (e) 765 g ↵
- (a) 99.41; (b) 2.27 g; (c) 3.5 g; (d) 222 kg; (e) 160.1 g ↵
- (a) 9.60 g; (b) 19.2 g; (c) 28.8 g ↵
- zirconium: 2.038 × 1023 atoms; 30.87 g; silicon: 2.038 × 1023 atoms; 9.504 g; oxygen: 8.151 × 1023 atoms; 21.66 g ↵
- AlPO4: 1.000 mol Al2Cl6: 1.994 mol Al2S3: 3.00 mol ↵
- 3.113 × 1025 C atoms ↵
- 0.865 servings, or about 1 serving. ↵
- 20.0 g H2O represents the least number of molecules since it has the least number of moles. ↵
- (a) % N = 82.24% % H = 17.76%; (b) % Na = 29.08% % S = 40.56% % O = 30.36%; (c) % Ca2+ = 38.76% ↵
- % NH3 = 38.2% ↵
- (a) CS2 (b) CH2O ↵
- C6H6 ↵
- Mg3Si2H3O8 (empirical formula), Mg6Si4H6O16 (molecular formula) ↵
- C15H15N3 ↵

