6.5 Naming Acids
Learning Objectives
By the end of this section, you will be able to:
- Define acid
- Name a binary acid and an oxyacid
There is one other group of compounds that is important to us – acids – and these compounds have interesting chemical properties. Initially, we will define an acid as an ionic compound of the H+ cation dissolved in water. To indicate that something is dissolved in water, we will use the phase label (aq) next to a chemical formula (where aq stands for “aqueous,” a word that describes something dissolved in water). If the formula does not have this label, then the compound is treated as a molecular compound rather than an acid.
Binary Acids
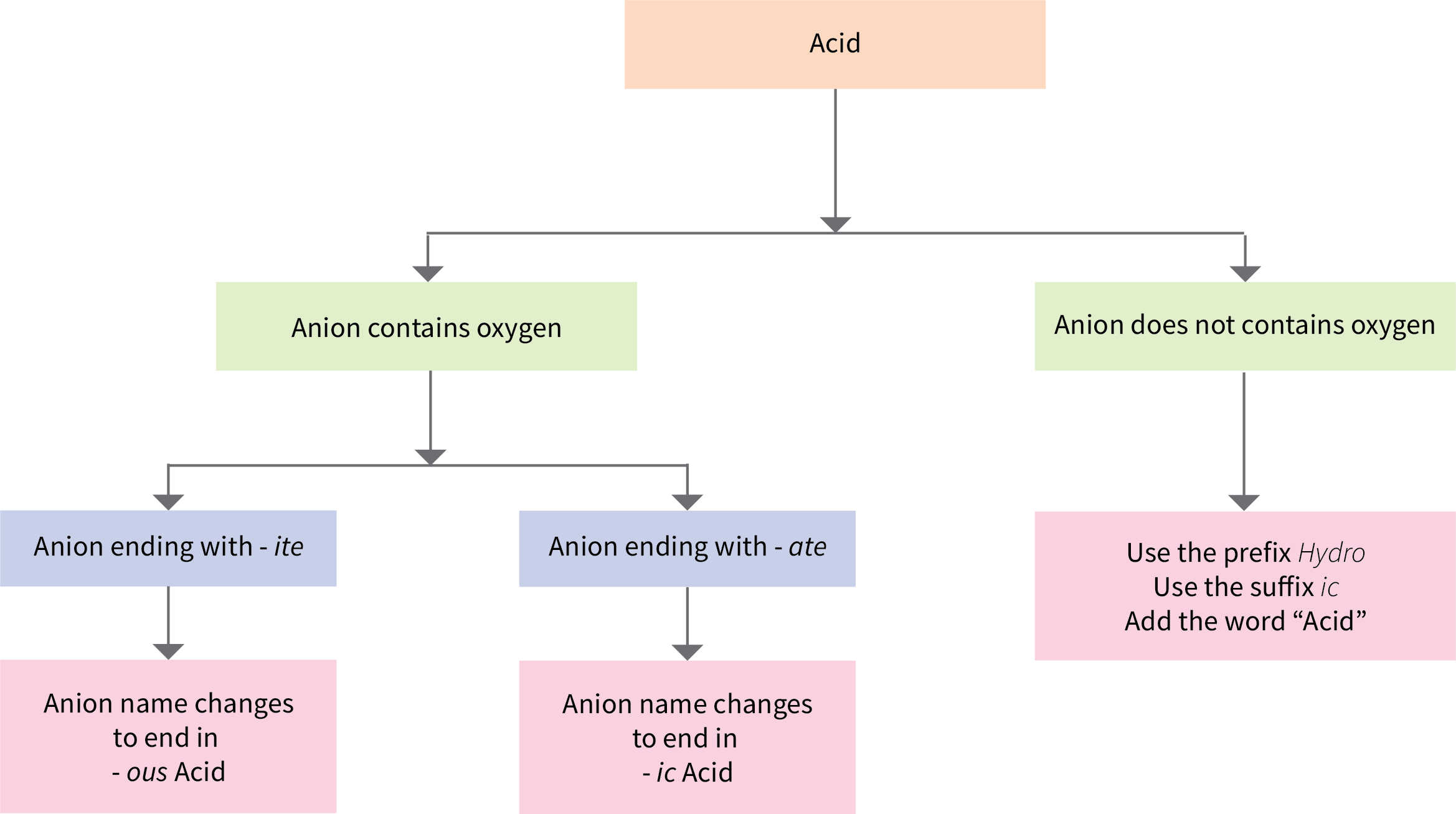
Some compounds containing hydrogen are members of an important class of substances known as acids. The chemistry of these compounds is explored in more detail in later chapters of this text, but for now, it will suffice to note that many acids release hydrogen ions, H+, when dissolved in water. To denote this distinct chemical property, a mixture of water with acid is given a name derived from the compound’s name. To indicate that something is dissolved in water, we will use the phrase label (aq) next to a chemical formula (where aq stands for “aqueous,” a word that describes something dissolved in water). If the formula does not have this label, then the compound is treated as a molecular compound rather than an acid. If the compound is a binary acid (comprised of hydrogen and one other nonmetallic element):
- The word “hydrogen” is changed to the prefix hydro-
- The other nonmetallic element name is modified by adding the suffix –ic
- The word “acid” is added as a second word
Figure 6.5a is a flowchart that summarizes naming acids.
For example, when the gas HCl (hydrogen chloride) is dissolved in water, the solution is called hydrochloric acid. Several other examples of this nomenclature are shown in Table 6.5a.
| Name of Gas | Name of Acid |
|---|---|
| HF(g), hydrogen fluoride | HF(aq), hydrofluoric acid |
| HCl(g), hydrogen chloride | HCl(aq), hydrochloric acid |
| HBr(g), hydrogen bromide | HBr(aq), hydrobromic acid |
| HI(g), hydrogen iodide | HI(aq), hydroiodic acid |
| H2S(g), hydrogen sulfide | H2S(aq), hydrosulfuric acid |
Oxyacids
Many compounds containing three or more elements (such as organic compounds or coordination compounds) are subject to specialized nomenclature rules that you will learn later. However, we will briefly discuss the important compounds known as oxyacids, compounds that contain hydrogen, oxygen, and at least one other element, and are bonded in such a way as to impart acidic properties to the compound (you will learn the details of this in a later chapter). Typical oxyacids consist of hydrogen combined with a polyatomic, oxygen-containing ion. To name oxyacids:
- Omit “hydrogen”
- Start with the root name of the anion
- Replace –ate with –ic, or –ite with –ous
- Add “acid”
For example, consider H2CO3 (which you might be tempted to call “hydrogen carbonate”). To name this correctly, “hydrogen” is omitted; the –ate of carbonate is replaced with –ic; and acid is added; its name is therefore carbonic acid. Other examples are given in Table 6.5b. There are some exceptions to the general naming method (e.g., H2SO4 is called sulfuric acid, not sulfic acid, and H2SO3 is sulfurous, not sulfous, acid).
| Formula | Anion Name | Acid Name |
|---|---|---|
| HC2H3O2 | acetate | acetic acid |
| HNO3 | nitrate | nitric acid |
| HNO2 | nitrite | nitrous acid |
| HClO4 | perchlorate | perchloric acid |
| H2CO3 | carbonate | carbonic acid |
| H2SO4 | sulfate | sulfuric acid |
| H2SO3 | sulfite | sulfurous acid |
| H3PO4 | phosphate | phosphoric acid |
Example 6.5a
Problems
Name each acid without consulting Table 6.5b.
- HBr
- H2SO4
Solutions
- As a binary acid, the acid’s name is hydro- + stem name + -ic acid. Because this acid contains a bromine atom, the name is hydrobromic acid.
- Because this acid is derived from the sulfate ion, the name of the acid is the stem of the anion name + -ic acid. The name of this acid is sulfuric acid.
Exercise 6.5a
All acids have some similar properties. For example, acids have a sour taste; in fact, the sour taste of some of our foods, such as citrus fruits and vinegar, is caused by the presence of acids in food. Many acids react with some metallic elements to form metal ions and elemental hydrogen. Acids make certain plant pigments change colours; indeed, the ripening of some fruits and vegetables is caused by the formation or destruction of excess acid in the plant. In a later chapter, we will explore the chemical behaviour of acids.
Acids are very prevalent in the world around us. We have already mentioned that citrus fruits contain acid; among other compounds, they contain citric acid, H3C6H5O7(aq). Oxalic acid, H2C2O4(aq), is found in spinach and other green leafy vegetables. Hydrochloric acid not only is found in the stomach (stomach acid) but also can be bought in hardware stores as a cleaner for concrete and masonry (under the common name muriatic acid). Phosphoric acid is an ingredient in some soft drinks.
Links to Interactive Learning Tools
Explore Naming Compounds Flow Chart from eCampusOntario H5P Studio.
Attribution & References
Except where otherwise noted, this page is adapted by Adrienne Richards from:
- “2.7 Chemical Nomenclature” In Chemistry 2e (OpenStax) by Paul Flowers, Klaus Theopold, Richard Langley, & William R. Robinson, licensed under CC BY 4.0.
- “4.3 Chemical Nomenclature” In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax)
- “Chapter 3: Ions and Ionic Compounds & Molecules and Molecular Compounds – Acids” In Introductory Chemistry: 1st Canadian Edition by David W. Ball and Jessica A. Key, licensed under CC BY NC SA 4.0. Access for free at Chemistry 2e (OpenStax).
Adaptations include combining section 2.7, 4.3 and Chapter 3.
-
- hydrofluoric acid
- nitrous acid
substance that produces H3O+ when dissolved in water
compound that contains hydrogen and one other element, bonded in a way that imparts acidic properties to the compound (ability to release H+ ions when dissolved in water)
compound that contains hydrogen, oxygen, and one other element, bonded in a way that imparts acidic properties to the compound (ability to release H+ ions when dissolved in water)

