6.3 Naming Binary Compounds
Learning Objectives
By the end of this section, you will be able to:
- Generate a proper name for an ionic compound
- Generate a proper name for a molecular compound
Nomenclature
Nomenclature, a collection of rules for naming things, is important in science and in many other situations. This module describes an approach that is used to name simple ionic and molecular compounds, such as NaCl, CaCO3, and N2O4. The simplest of these are binary compounds, those containing only two elements, but we will also consider how to name ionic compounds containing polyatomic ions, and one specific, very important class of compounds known as acids (subsequent chapters in this text will focus on these compounds in great detail). We will limit our attention here to inorganic compounds, compounds that are composed principally of elements other than carbon, and will follow the nomenclature guidelines proposed by IUPAC. The rules for organic compounds, in which carbon is the principal element, will be introduced in a later chapter on organic chemistry.
Naming Binary Ionic Compounds
Naming ionic compounds is simple: combine the name of the cation and the name of the anion, in both cases omitting the word ion. Do not use numerical prefixes if there is more than one ion necessary to balance the charges. NaCl is sodium chloride, a combination of the name of the cation (sodium) and the anion (chloride). MgO is magnesium oxide. MgCl2 is magnesium chloride—not magnesium dichloride.
In naming ionic compounds whose cations can have more than one possible charge, we must also include the charge, in parentheses and in roman numerals, as part of the name. Hence FeS is iron(II) sulfide, while Fe2S3 is iron(III) sulfide. Again, no numerical prefixes appear in the name. The number of ions in the formula is dictated by the need to balance the positive and negative charges.
Example 6.3a
Problems
Name each ionic compound.
- CaCl2
- AlF3
Solutions
- Using the names of the ions, this ionic compound is named calcium chloride. It is not calcium(II) chloride because calcium forms only one cation when it forms an ion, and it has a characteristic charge of 2+.
- The name of this ionic compound is aluminum fluoride.
Exercise 6.3a
Compounds Containing a Metal Ion with a Variable Charge
Most transition metals can form two or more cations with different charges.
There are two ways to make this distinction. In the simpler, more modern approach, called the Stock system, an ion’s positive charge is indicated by a roman numeral in parentheses after the element name, followed by the word ion. Thus Fe2+ is called the iron(II) ion, while Fe3+ is called the iron(III) ion. This system is used only for elements that form more than one common positive ion. We do not call the Na+ ion the sodium(I) ion because (I) is unnecessary. Sodium forms only a 1+ ion, so there is no ambiguity about the name sodium ion.
| Element | Stem | Charge | Modern Name | Common Name |
|---|---|---|---|---|
| iron | ferr- | 2+ | iron(II) ion | ferrous ion |
| iron | ferr- | 3+ | iron(III) ion | ferric ion |
| copper | cupr- | 1+ | copper(I) ion | cuprous ion |
| copper | cupr- | 2+ | copper(II) ion | cupric ion |
| tin | stann- | 2+ | tin(II) ion | stannous ion |
| tin | stann- | 4+ | tin(IV) ion | stannic ion |
| lead | plumb- | 2+ | lead(II) ion | plumbous ion |
| lead | plumb- | 4+ | lead(IV) ion | plumbic ion |
| chromium | chrom- | 2+ | chromium(II) ion | chromous ion |
| chromium | chrom- | 3+ | chromium(III) ion | chromic ion |
| gold | aur- | 1+ | gold(I) ion | aurous ion |
| gold | aur- | 3+ | gold(III) ion | auric ion |
The second system, called the common system, is not conventional but is still prevalent and used in the health sciences. This system recognizes that many metals have two common cations. The common system uses two suffixes (-ic and –ous) that are appended to the stem of the element name. The -ic suffix represents the greater of the two cation charges, and the -ous suffix represents the lower one. In many cases, the stem of the element name comes from the Latin name of the element.
Source: “3.4 Ionic Nomenclature” In The Basics of General, Organic, and Biological Chemistry by Saylor Academy, licensed under CC BY-NC-SA 3.0.
Compounds of these metals with nonmetals are named with the same method as compounds in the first category, except the charge of the metal ion is specified by a Roman numeral in parentheses after the name of the metal. The charge of the metal ion is determined from the formula of the compound and the charge of the anion. For example, consider binary ionic compounds of iron and chlorine. Iron typically exhibits a charge of either 2+ or 3+, and the two corresponding compound formulas are FeCl2 and FeCl3. The simplest name, “iron chloride,” will, in this case, be ambiguous, as it does not distinguish between these two compounds. In cases like this, the charge of the metal ion is included as a Roman numeral in parentheses immediately following the metal name. These two compounds are then unambiguously named iron(II) chloride and iron(III) chloride, respectively. Other examples are provided in Table 6.3b.
| Transition Metal Ionic Compound | Name |
|---|---|
| FeCl3 | iron(III) chloride |
| Hg2O | mercury(I) oxide |
| HgO | mercury(II) oxide |
| Cu3(PO4)2 | copper(II) phosphate |
Table source: General Chemistry 1 & 2 , a derivative of Chemistry (Open Stax), CC BY 4.0
Out-of-date nomenclature used the suffixes –ic and –ous to designate metals with higher and lower charges, respectively: Iron(III) chloride, FeCl3, was previously called ferric chloride, and iron(II) chloride, FeCl2, was known as ferrous chloride. Though this naming convention has been largely abandoned by the scientific community, it remains in use by some segments of industry. For example, you may see the words stannous fluoride on a tube of toothpaste. This represents the formula SnF2, which is more properly named tin(II) fluoride. The other fluoride of tin is SnF4, which was previously called stannic fluoride but is now named tin(IV) fluoride.
Example 6.3b
Naming Ionic Compounds
Name the following ionic compounds, which contain a metal that can have more than one ionic charge:
- Fe2S3
- CuSe
- GaN
- CrCl3
- Ti2(SO4)3
Solution
The anions in these compounds have a fixed negative charge (S2−, Se2− , N3−, Cl−, and SO42−), and the compounds must be neutral. Because the total number of positive charges in each compound must equal the total number of negative charges, the positive ions must be Fe3+, Cu2+, Ga3+, Cr3+, and Ti3+. These charges are used in the names of the metal ions:
- iron(III) sulfide
- copper(II) selenide
- gallium(III) nitride
- chromium(III) chloride
- titanium(III) sulfate
Exercise 6.3b
Write the formulas of the following ionic compounds:
- chromium(III) phosphide
- mercury(II) sulfide
- manganese(II) phosphate
- copper(I) oxide
- chromium(VI) fluoride
Check Your Answer[2]
Erin Brockovich and Chromium Contamination
In the early 1990s, legal file clerk Erin Brockovich discovered a high rate of serious illnesses in the small town of Hinckley, California. Her investigation eventually linked the illnesses to groundwater contaminated by Cr(VI) (Figure 6.3a) used by Pacific Gas & Electric (PG&E) to fight corrosion in a nearby natural gas pipeline. As dramatized in the film Erin Brokovich (for which Julia Roberts won an Oscar), Erin and lawyer Edward Masry sued PG&E for contaminating the water near Hinckley in 1993. The settlement they won in 1996—$333 million—was the largest amount ever awarded for a direct-action lawsuit in the US at that time.
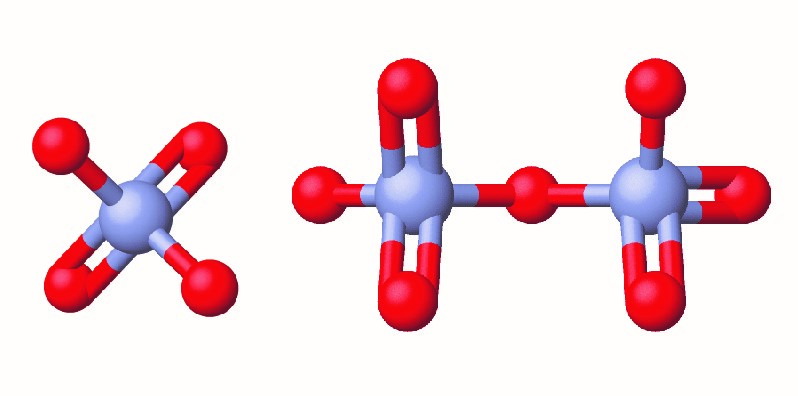
Chromium compounds are widely used in industry, such as for chrome plating, in dye-making, as preservatives, and to prevent corrosion in cooling tower water, as occurred near Hinckley. In the environment, chromium exists primarily in either the Cr(III) or Cr(VI) forms. Cr(III), an ingredient of many vitamin and nutritional supplements, forms compounds that are not very soluble in water, and it has low toxicity. But Cr(VI) is much more toxic and forms compounds that are reasonably soluble in water. Exposure to small amounts of Cr(VI) can lead to damage of the respiratory, gastrointestinal, and immune systems, as well as the kidneys, liver, blood, and skin.
Despite cleanup efforts, Cr(VI) groundwater contamination remains a problem in Hinckley and other locations across the globe. A 2010 study by the Environmental Working Group found that of 35 US cities tested, 31 had higher levels of Cr(VI) in their tap water than the public health goal of 0.02 parts per billion set by the California Environmental Protection Agency.
Naming Molecular Compounds
How do you know whether a formula—and by extension, a name—is for a molecular compound or for an ionic compound? Molecular compounds form between two or more nonmetals, while ionic compounds form between metals and nonmetals.
There are many substances that exist as two or more atoms connected together so strongly that they behave as a single particle. These multiatom combinations are called molecules. A molecule is the smallest part of a substance that has the physical and chemical properties of that substance. In some respects, a molecule is similar to an atom. A molecule, however, is composed of more than one atom.
Some elements exist naturally as molecules. For example, hydrogen and oxygen exist as two-atom molecules. Other elements also exist naturally as diatomic molecules (Table 6.3c). As with any molecule, these elements are labeled with a molecular formula, a formal listing of what and how many atoms are in a molecule. (Sometimes only the word formula is used, and its meaning is inferred from the context.) For example, the molecular formula for elemental hydrogen is H2, with H being the symbol for hydrogen and the subscript 2 implying that there are two atoms of this element in the molecule. Other diatomic elements have similar formulas: O2, N2, and so forth. Other elements exist as molecules—for example, sulfur normally exists as an eight-atom molecule, S8, while phosphorus exists as a four-atom molecule, P4 (see Figure 6.3b). Otherwise, we will assume that elements exist as individual atoms, rather than molecules. It is assumed that there is only one atom in a formula if there is no numerical subscript on the right side of an element’s symbol.
| Name | Symbol |
|---|---|
| hydrogen | H2 |
| oxygen | O2 |
| nitrogen | N2 |
| fluorine | F2 |
| chlorine | Cl2 |
| bromine | Br2 |
| iodine | I2 |
Table source: “Molecules and Chemical Nomenclature” In Introductory Chemistry: 1st Canadian Edition by David W. Ball and Jessica A. Key, CC BY-NC-SA 4.0.
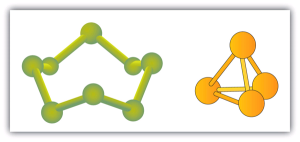
Figure 6.3b shows two examples of how we will be representing molecules in this text. An atom is represented by a small ball or sphere, which generally indicates where the nucleus is in the molecule. A cylindrical line connecting the balls represents the connection between the atoms that make this collection of atoms a molecule. This connection is called a chemical bond.
Many compounds exist as molecules. In particular, when nonmetals connect with other nonmetals, the compound typically exists as molecules.
By following the rules of nomenclature, each and every compound has its own unique name, and each name refers to one and only one compound. Here, we will start with relatively simple molecules that have only two elements in them, the so-called binary compounds:
Step 1: Identify the elements in the molecule from its formula.
Step 2: Begin the name with the element name of the first element. If there is more than one atom of this element in the molecular formula, use a numerical prefix to indicate the number of atoms, as listed in Table 6.3c “Numerical Prefixes Used in Naming Molecular Compounds”. Do not use the prefix mono- if there is only one atom of the first element.
| Number of Atoms of Element | Prefix |
|---|---|
| 1 | mono- |
| 2 | di- |
| 3 | tri- |
| 4 | tetra- |
| 5 | penta- |
| 6 | hexa- |
| 7 | hepta- |
| 8 | octa- |
| 9 | nona- |
| 10 | deca- |
Step 3: Name the second element by using three pieces:
-
- a numerical prefix indicating the number of atoms of the second element, plus
- the stem of the element name (e.g., ox for oxygen, chlor for chlorine, etc.), plus
- the suffix –ide.
Step 4: Combine the two words, leaving a space between them.
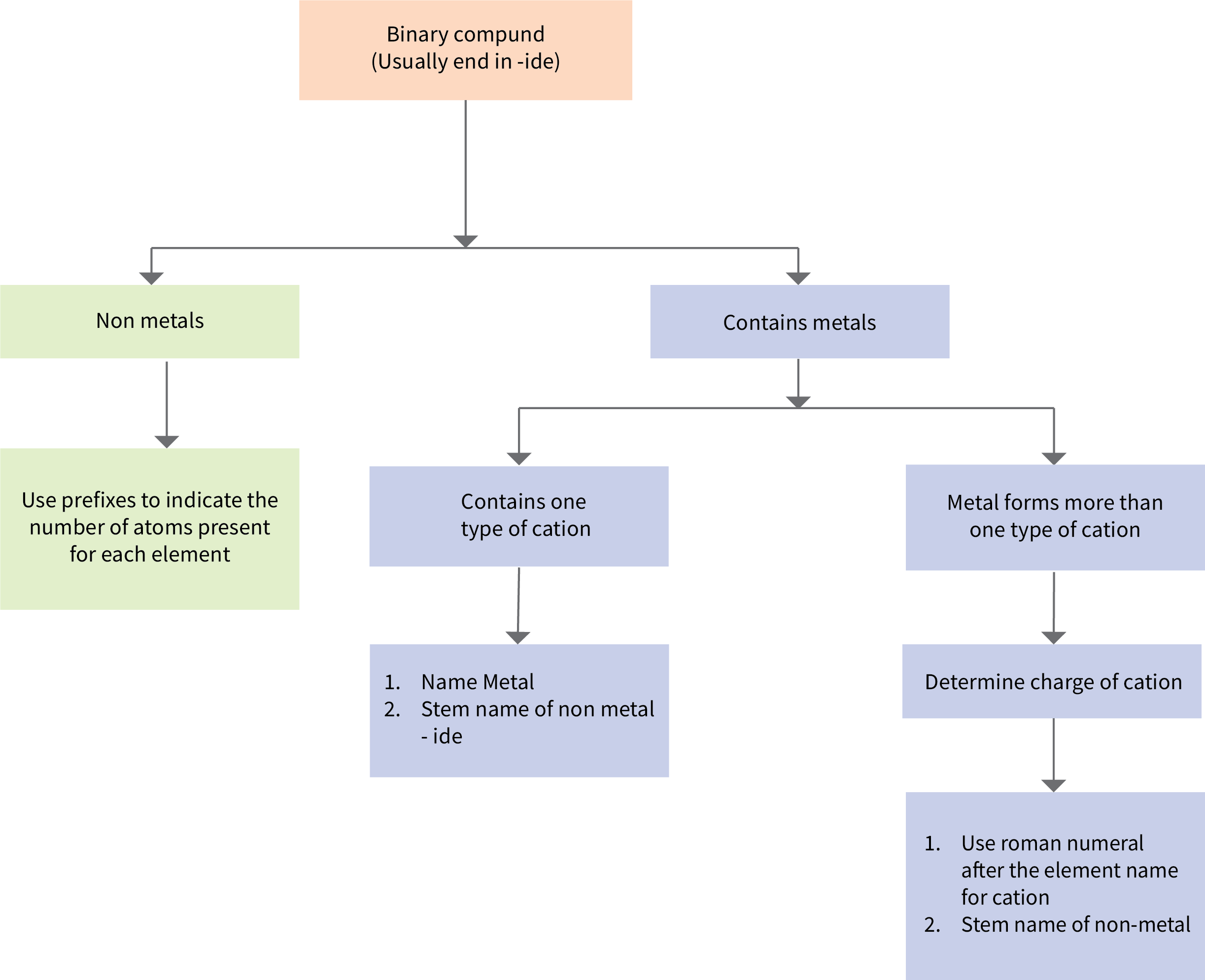
Figure 6.3c provides a flowchart summary for naming binary compounds.
Let us see how these steps work for a molecule whose molecular formula is SO2, which has one sulfur atom and two oxygen atoms—this completes step 1. According to step 2, we start with the name of the first element—sulfur. Remember, we don’t use the mono- prefix for the first element. Now for step 3, we combine the numerical prefix di- (see Table 6.3c) with the stem ox- and the suffix -ide, to make dioxide. Bringing these two words together, we have the unique name for this compound: sulfur dioxide.
Why all this trouble? There is another common compound consisting of sulfur and oxygen whose molecular formula is SO3, so the compounds need to be distinguished. SO3 has three oxygen atoms in it, so it is a different compound with different chemical and physical properties. The system of chemical nomenclature is designed to give this compound its own unique name. Its name, if you go through all the steps, is sulfur trioxide. Different compounds have different names.
In some cases, when a prefix ends in a or o and the element name begins with o we drop the a or o on the prefix. So we see monoxide or pentoxide rather than monooxide or pentaoxide in molecule names.
One great thing about this system is that it works both ways. From the name of a compound, you should be able to determine its molecular formula. Simply list the element symbols, with a numerical subscript if there is more than one atom of that element, in the order of the name (we do not use a subscript 1 if there is only one atom of the element present; 1 is implied). From the name nitrogen trichloride, you should be able to get NCl3 as the formula for this molecule. From the name diphosphorus pentoxide, you should be able to get the formula P2O5 (note the numerical prefix on the first element, indicating there is more than one atom of phosphorus in the formula).
Example 6.3c
Problems
Name each molecule.
- PF3
- CO
- Se2Br2
Solutions
- A molecule with a single phosphorus atom and three fluorine atoms is called phosphorus trifluoride.
- A compound with one carbon atom and one oxygen atom is properly called carbon monoxide, not carbon monooxide.
- There are two atoms of each element, selenium and bromine. According to the rules, the proper name here is diselenium dibromide.
Exercise 6.3c
Example 6.3d
Problems
Give the formula for each molecule.
- carbon tetrachloride
- silicon dioxide
- trisilicon tetranitride
Solutions
- The name carbon tetrachloride implies one carbon atom and four chlorine atoms, so the formula is CCl4.
- The name silicon dioxide implies one silicon atom and two oxygen atoms, so the formula is SiO2.
- We have a name that has numerical prefixes on both elements. Tri- means three, and tetra- means four, so the formula of this compound is Si3N4.
Exercise 6.3d
Some simple molecules have common names that we use as part of the formal system of chemical nomenclature. For example, H2O is given the name water, not dihydrogen monoxide. NH3 is called ammonia, while CH4 is called methane. We will occasionally see other molecules that have common names; we will point them out as they occur.
Links to Interactive Learning Tools
Practice Names to Formulas 1 by the Physics Classroom.
Explore Ion and Formula Writing from eCampusOntario H5P Studio.
Explore Naming Compounds Flow Chart from eCampusOntario H5P Studio.
Attribution & References
Except where otherwise noted, this page is adapted by Adrienne Richards from”4.3 Chemical Nomenclature” In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax)
- “Naming Molecular Compounds” section is adapted from Chapter 3: Ions and Ionic Compounds & Molecules and Chemical Nomenclature In Introductory Chemistry: 1st Canadian Edition by David W. Ball and Jessica A. Key, licensed under CC BY-NC-SA 4.0.
system of rules for naming objects of interest
compound containing two different elements.
substance that produces H3O+ when dissolved in water
The system of indicating a cation’s charge with roman numerals.

