15.2 Electrolytes
Learning Objectives
- Define electrolyte and give examples of electrolytes
- Relate electrolyte strength to solute-solvent attractive forces
Pure water (purified water) is a pure substance made up of just three atoms: two hydrogen and one oxygen atom, H2O. Since pure water has no constituents other than these atoms, it does not have any taste or smell, and it doesn’t conduct electricity on its own. However, water can become a medium for conducting electricity. When ionic substances (salts) are dissolved in water, they undergo either a physical or a chemical change that yields free ions moving independently in the aqueous solution. This feature permits them to carry positive or negative electrical charges from one place to another and the solution can conduct an electrical current. These substances constitute an important class of compounds called electrolytes. Salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. Substances that do not yield ions when dissolved are called nonelectrolytes. If the physical or chemical process that generates the ions is essentially 100% efficient (all of the dissolved compound yields ions), then the substance is known as a strong electrolyte. If only a relatively small fraction of the dissolved substance undergoes the ion-producing process, it is called a weak electrolyte.
Substances may be identified as strong, weak, or nonelectrolytes by measuring the electrical conductance of an aqueous solution containing the substance. To conduct electricity, a substance must contain freely mobile, charged species. Most familiar is the conduction of electricity through metallic wires, in which case the mobile, charged entities are electrons. Solutions may also conduct electricity if they contain dissolved ions, with conductivity increasing as ion concentration increases. Applying a voltage to electrodes immersed in a solution permits assessment of the relative concentration of dissolved ions, either quantitatively, by measuring the electrical current flow, or qualitatively, by observing the brightness of a light bulb included in the circuit (Figure 15.2a).
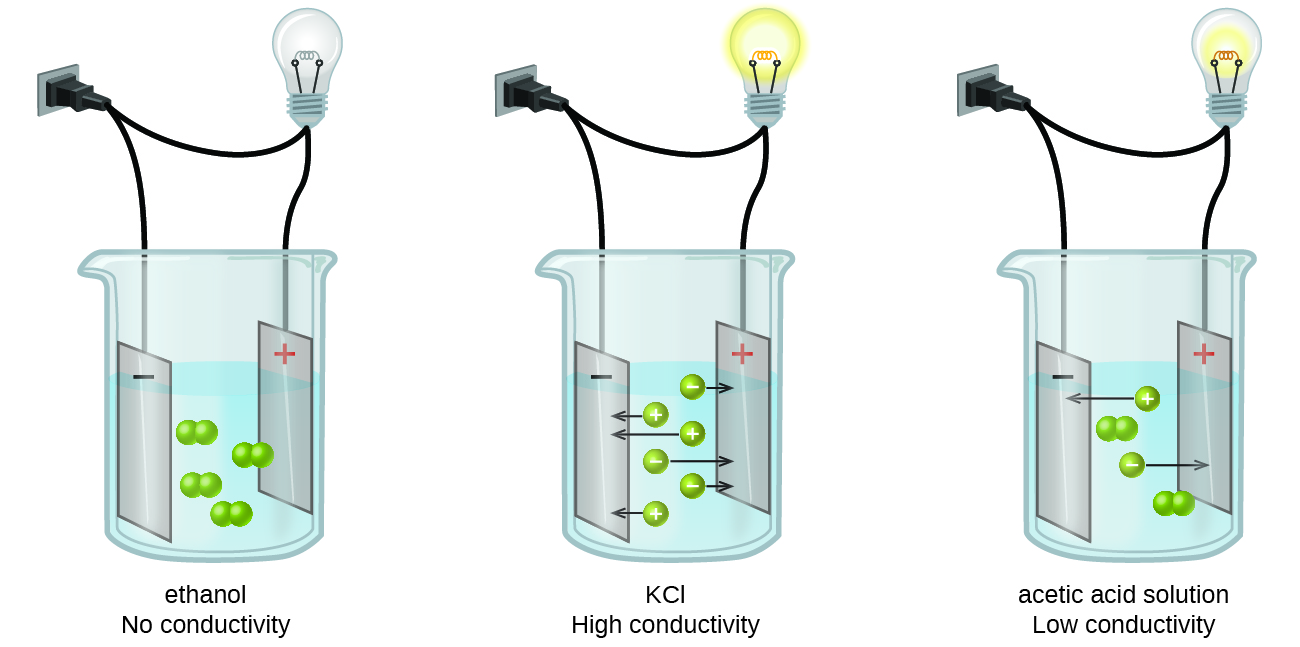
Ionic Electrolytes
Water and other polar molecules are attracted to ions, as shown in Figure 15.2b. The electrostatic attraction between an ion and a molecule with a dipole is called an ion-dipole attraction. These attractions play an important role in the dissolution (ionization) of ionic compounds in water.
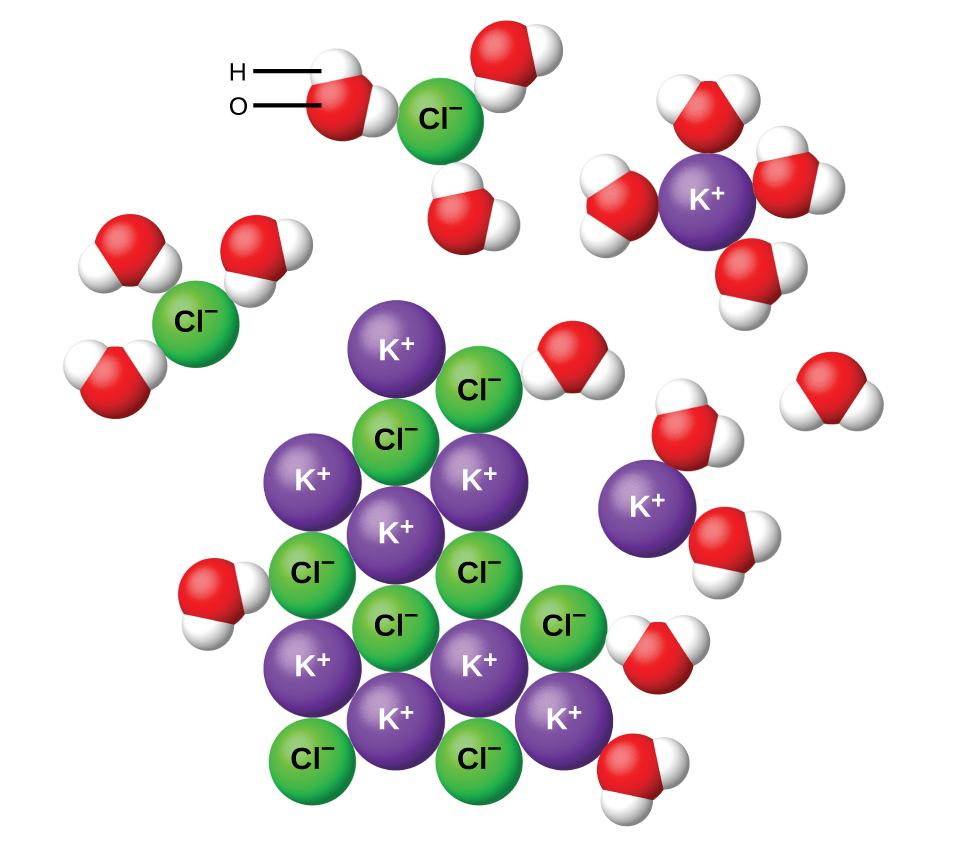
When ionic compounds dissolve in water, the ions in the solid separate and disperse uniformly throughout the solution because water molecules surround and solvate the ions, reducing the strong electrostatic forces between them. This process represents a physical change known as dissociation, which was discussed in the previous chapter – Solutions. Under most conditions, ionic compounds will dissociate nearly completely when dissolved, and so they are classified as strong electrolytes.
Let us consider what happens at the microscopic level when we add solid KCl to water. Ion-dipole forces attract the positive (hydrogen) end of the polar water molecules to the negative chloride ions at the surface of the solid, and they attract the negative (oxygen) ends to the positive potassium ions. The water molecules penetrate between individual K+ and Cl− ions and surround them, reducing the strong interionic forces that bind the ions together and letting them move off into solution as solvated ions, as Figure 15.2b shows. The reduction of the electrostatic attraction permits the independent motion of each hydrated ion in a dilute solution, resulting in an increase in the disorder of the system as the ions change from their fixed and ordered positions in the crystal to mobile and much more disordered states in solution. This increased disorder is responsible for the dissolution of many ionic compounds, including KCl, which dissolve with the absorption of heat.
In other cases, the electrostatic attractions between the ions in a crystal are so large, or the ion-dipole attractive forces between the ions and water molecules are so weak, that the increase in disorder cannot compensate for the energy required to separate the ions, and the crystal is insoluble. Such is the case for salt compounds such as calcium carbonate (limestone), calcium phosphate (the inorganic component of bone), and iron oxide (rust).
Covalent Electrolytes
As mentioned previously, pure water is an extremely poor conductor of electricity because it is only very slightly ionized—only about two out of every 1 billion molecules ionize at 25 °C. The ionization of water will be discussed in more depth later in this chapter and these concepts will be important in understanding the behaviours of acids and bases. In brief, water ionizes when one molecule of water gives up a proton to another molecule of water, yielding hydronium and hydroxide ions.
[latex]\text{H}_2\text{O}(l)\;+\;\text{H}_2\text{O}(l)\;{\leftrightharpoons}\;\text{H}_3\text{O}^{+}(aq)\;+\;\text{OH}^{-}(aq)[/latex]
In some cases, we find that solutions prepared from covalent compounds conduct electricity because the solute molecules react chemically with the solvent to produce ions. For example, pure hydrogen chloride is a gas consisting of covalently bonded HCl molecules. This gas contains no ions. Hydrogen chloride gas is very soluble in water and is dissolved in water to prepare hydrochloric acid. When we dissolve hydrogen chloride gas in water, we find that the solution is a very good conductor. The water molecules play an essential part in forming ions. However, it is important to note that solutions of hydrogen chloride in many other solvents, such as benzene, do not conduct electricity and do not contain ions.
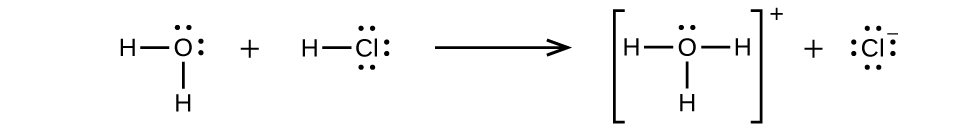
Hydrogen chloride gas dissolves in water and the ions react with water, transferring H+ ions to form hydronium ions (H3O+) and chloride ions (Cl−). This illustrates the ionization of a strong acid.
This reaction is essentially 100% complete for HCl (i.e., it is a strong acid and, consequently, a strong electrolyte). Likewise, weak acids and bases that only react partially generate relatively low concentrations of ions when dissolved in water and are classified as weak electrolytes. You will learn more about classifying the strength of acids and bases in later chapters.
For a summary Watch Aqueous Solutions, Dissolving, and Solvation (14min 6sec).
Video Source: DeWitt, T. (2021, May 11). Aqueous solutions, dissolving, and solvation [Video]. YouTube.
Attribution & References
Except where otherwise noted, this page is adapted by Jackie MacDonald from “11.2 Electrolytes” In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax). / Adaptations and additions were made to content in this section were made or student comprehension.
substances that produce ions when dissolved in water and whose aqueous solutions can conduct electricity
are substances that do not readily ionize (do not produce ions) when dissolved in aqueous (water) solution or in molten state and are poor conductors of electricity.
a substance that dissociates or ionizes completely when dissolved in water and can conduct electricity.
a substance that ionizes only partially when dissolved in water, thus producing an aqueous solution that conducts electricity poorly.
electrostatic attraction between an ion and a polar molecule
physical process accompanying the dissolution of an ionic compound in which the compound’s constituent ions are solvated and dispersed throughout the solution

