16.7 Buffers
Learning Objectives
By the end of this section, you will be able to:
- Define what a buffer is and describe how it reacts with an acid or a base
Weak acids are relatively common, even in the foods we eat. But we occasionally come across a strong acid or base, such as stomach acid, that has a strongly acidic pH of 1–2. By definition, strong acids and bases can produce a relatively large amount of hydrogen or hydroxide ions and, as a consequence, have marked chemical activity. In addition, very small amounts of strong acids and bases can change the pH of a solution very quickly. If 1 mL of stomach acid [which we will approximate as 0.05 M HCl(aq)] is added to the bloodstream, and if no correcting mechanism is present, the pH of the blood would go from about 7.4 to about 4.9 — a pH that is not conducive to life. Fortunately, the body has a mechanism for minimizing such dramatic pH changes. This mechanism involves a buffer, a solution that resists dramatic changes in pH.
Watch Buffers, the Acid Rain Slayer: Crash Course Chemistry #31 (11min 40s).
Video Source: Crash Course. (2013, September 16). Buffers, the acid rain slayer: Crash course Chemistry #31 [Video]. YouTube.
Buffers resist dramatic changes in pH by being composed of certain pairs of solutes: either a weak acid plus a salt derived from that weak acid, or a weak base plus a salt of that weak base. For example, a buffer can be composed of dissolved acetic acid (HC2H3O2, a weak acid) and sodium acetate (NaC2H3O2, a salt derived from that acid). Another example of a buffer is a solution containing ammonia (NH3, a weak base) and ammonium chloride (NH4Cl, a salt derived from that base).
Characteristics of a Good Buffer
Good buffering systems have the following characteristics:
- The solution contains a weak acid and its conjugate base OR a weak base and its conjugate acid
- The buffer resists changes in pH by reacting with added acid or base, so these ions do not accumulate.
- Any added acid reacts with the conjugate base to resist pH changes
- Any added base reacts with the conjugate acid to resist pH changes
Buffers cannot be made from a strong acid (or strong base) and its conjugate since these solutions ionize completely in water. Also take note, water is not a buffer.
Source: “Characteristics of a Good Buffer” by Jackie MacDonald, CC BY-NC-4.0
How Buffers Work
Let’s consider an acetic acid – sodium acetate buffer to demonstrate how buffers work. If a strong base – a source of OH−(aq) ions – is added to the buffer solution, those hydroxide ions will react with the acetic acid in an acid-base reaction:
HC2H3O2(aq) + OH−(aq) → H2O(l) + C2H3O2–(aq)
Rather than changing the pH dramatically by making the solution basic, the added hydroxide ions react to make water, and the pH does not change much.
If a strong acid – a source of H+ ions – is added to the buffer solution, the H+ ions will react with the anion from the salt. Because HC2H3O2 is a weak acid, it is not ionized much. This means that if lots of hydrogen ions and acetate ions (from sodium acetate) are present in the same solution, they will come together to make acetic acid:
H+(aq) + C2H3O2−(aq) → HC2H3O2(aq)
Rather than changing the pH dramatically and making the solution acidic, the added hydrogen ions react to make molecules of a weak acid.
In chemistry texts and sources, you may have noticed that H+ and H3O+ are used interchangeably in contexts when the proton donor-acceptor mechanism does not need to be emphasized. Since it is easier to write the H+ proton, chemists often use it to represent acid-base reactions or to explain general concepts in buffering systems. Thus, it is permissible to talk about “hydrogen ions” and use the formula H+ in writing chemical equations as long as you remember that they are not to be taken literally in the context of aqueous solutions.
Source: “The Hydronium Ion” by Stephen Lower & Avneet Kahlon In Acids and Bases in Aqueous Solutions, licensed under CC BY 3.0.
Figure 16.7a illustrates both actions of the acetic acid – sodium acetate buffer.
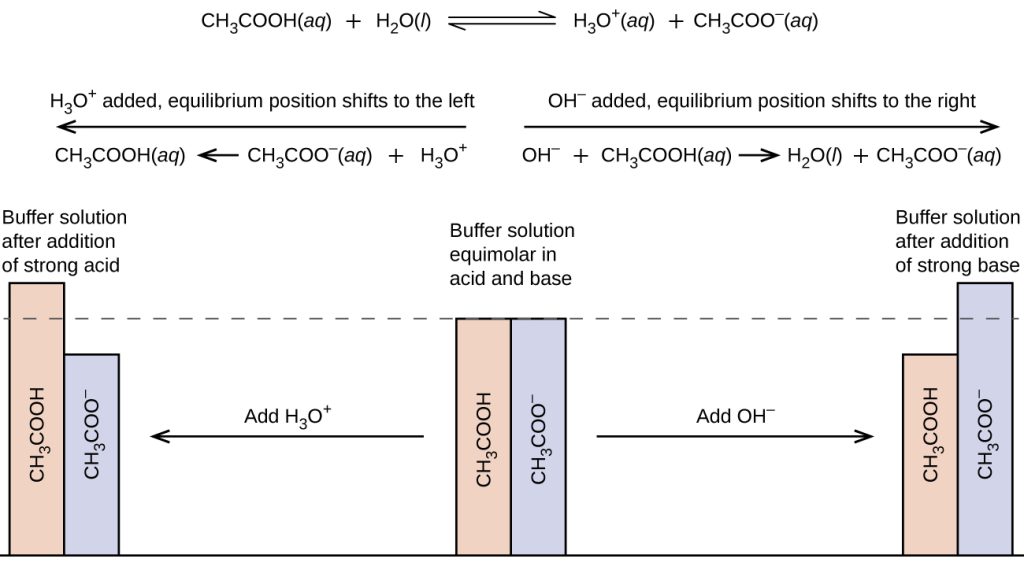
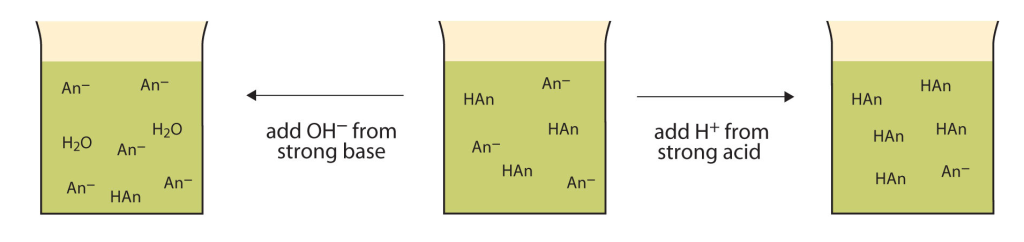
Figure 16.7b illustrates a basic summary of the action of buffers when small amounts of strong base and acid is added.
Buffers made from weak bases and salts of weak bases act similarly. For example, in a buffer containing NH3 and NH4Cl, ammonia molecules can react with any excess hydrogen ions introduced by strong acids:
NH3(aq) + H+(aq) → NH4+(aq)
while the ammonium ion, NH4+(aq) can react with any hydroxide ions introduced by strong bases:
NH4+(aq) + OH−(aq) → NH3(aq) + H2O(l)
Example 16.7a
Which solute combinations can make a buffer solution? Assume that all are aqueous solutions.
- HCHO2 and NaCHO2
- HCl and NaCl
- CH3NH2 and CH3NH3Cl
- NH3 and NaOH
Solution
- Formic acid (HCHO2) is a weak acid, while NaCHO2 is the salt made from the anion of the weak acid – the formate ion (CHO2−). The combination of these two solutes would make a buffer solution.
- Hydrochloric acid (HCl) is a strong acid, not a weak acid, so the combination of these two solutes would not make a buffer solution.
- Methylamine (CH3NH2) is like ammonia with one of its hydrogen atoms substituted with a CH3 (methyl) group. Because it is not on our list of strong bases, we can assume that it is a weak base. The compound CH3NH3Cl is a salt made from that weak base, so the combination of these two solutes would make a buffer solution.
- Ammonia (NH3) is a weak base, but NaOH is a strong base. The combination of these two solutes would not make a buffer solution.
Exercise 16.7a
Which solute combinations can make a buffer solution? Assume that all are aqueous solutions.
- NaHCO3 and NaCl
- H3PO4 and NaH2PO4
- NH3 and (NH4)3PO4
- NaOH and NaCl
Check Your Answer[1]
Buffers work well only for limited amounts of added strong acid or base. Once either solute is all reacted, the solution is no longer a buffer, and rapid changes in pH may occur. We say that a buffer has a certain capacity. Buffers that have more solute dissolved in them to start with have larger capacities, as might be expected.
Human blood has a buffering system to minimize extreme changes in pH. One buffer in blood is based on the presence of HCO3− and H2CO3 [H2CO3 is another way to write CO2(aq)]. With this buffer present, even if some stomach acid were to find its way directly into the bloodstream, the change in the pH of blood would be minimal. Inside many of the body’s cells, there is a buffering system based on phosphate ions.
Buffered Aspirin
Many people are aware of the concept of buffers from buffered aspirin. Aspirin is well known as a pain reliever and fever reducer. Buffered aspirin contains aspirin (acetylsalicylic acid) and also has magnesium carbonate, calcium carbonate, magnesium oxide, or some other salt. The salt regulates the acidity of the aspirin to minimize its acidic side effects in the stomach. The salt acts like a base, while aspirin is itself a weak acid due to its carboxylic acid group. The H atom in that group can be donated, and therefore, aspirin can act as a Brønsted-Lowry acid. Figure 16.7c and 16.7d show the molecular structure of aspirin in 3D and 2D.
Links to Interactive Learning Tools
Explore Learn the Basics about Buffers from eCampusOntario H5P Studio.
Attribution & References
Except where otherwise noted, this page is adapted by Jackie MacDonald from a section in “14.10: Buffers- Solutions that Resist pH Change” In Map: Introductory Chemistry (Tro) by Marisa Alviar-Agnew & Henry Agnew, shared under a CK-12 license.
References
Stephen Lower & Avneet Kahlon. (2022, August 10). The Hydronium Ion. Chemistry LibreTexts.
-
- No; NaHCO3 and NaCl are not acid/base conjugate pairs;
- Yes;
- Yes; H3PO4 is a weak acid and NaH2PO4 is a salt of its conjugate base;
- No - NaOH is a strong base, a buffer requires a weak base or acid and its conjugate. ↵

