Chapter 2 – Review
2.1 Measurements
- Is one liter about an ounce, a pint, a quart, or a gallon?
- Is a meter about an inch, a foot, a yard, or a mile?
Check answers: [1] - Indicate the SI base units or derived units that are appropriate for the following measurements:
- the length of a marathon race (26 miles 385 yards)
- the mass of an automobile
- the volume of a swimming pool
- the speed of an airplane
- the density of gold
- the area of a football field
- the maximum temperature at the South Pole on April 1, 1913
- Indicate the SI base units or derived units that are appropriate for the following measurements:
- the mass of the moon
- the distance from Dallas to Oklahoma City
- the speed of sound
- the density of air
- the temperature at which alcohol boils
- the area of the state of Delaware
- the volume of a flu shot or a measles vaccination
Check answers: [2]
-
Give the name and symbol of the prefixes used with SI units to indicate multiplication by the following exact quantities.
-
103
-
10−2
-
0.1
-
10−3
-
1,000,000
-
0.000001
-
-
Give the name of the prefix and the quantity indicated by the following symbols that are used with SI base units.
-
c
-
d
-
G
-
k
-
m
-
n
-
p
-
T
Check answers: [3]
-
-
A large piece of jewelry has a mass of 132.6 g. A graduated cylinder initially contains 48.6 mL water. When the jewelry is submerged in the graduated cylinder, the total volume increases to 61.2 mL.
- Determine the density of this piece of jewelry.
- Assuming that the jewelry is made from only one substance, what substance is it likely to be? Explain.
-
Visit this density simulation and click the “turn fluid into water” button to adjust the density of liquid in the beaker to 1.00 g/mL.
- Use the water displacement approach to measure the mass and volume of the unknown material (select the green block with question marks).
- Use the measured mass and volume data from step (a) to calculate the density of the unknown material.
- Link out to the link provided.
-
Assuming this material is a copper-containing gemstone, identify its three most likely identities by comparing the measured density to the values tabulated in this gemstone density guide.
- How are mass and density related for blocks of the same volume?
Check answers: [4]
- Visit this density simulation and click the “reset” button to ensure all simulator parameters are at their default values.
- Use the water displacement approach to measure the mass and volume of the red block.
- Use the measured mass and volume data from step (a) to calculate the density of the red block.
- Use the vertical green slide control to adjust the fluid density to values well above, then well below, and finally nearly equal to the density of the red block, reporting your observations.
- Visit this density simulation and click the “turn fluid into water” button to adjust the density of liquid in the beaker to 1.00 g/mL. Change the block material to foam, and then wait patiently until the foam block stops bobbing up and down in the water.
- The foam block should be floating on the surface of the water (that is, only partially submerged). What is the volume of water displaced?
- Use the water volume from part (a) and the density of water (1.00 g/mL) to calculate the mass of water displaced.
- Remove and weigh the foam block. How does the block’s mass compare to the mass of displaced water from part (b)? Check answers: [5]
2.2 Measurement Uncertainty, Accuracy, and Precision
- Express each of the following numbers in scientific notation with correct significant figures:
- 711.0
- 0.03344
- 547.9
- 22086
- 1000.00
- 0.0000000651
- 0.007157
- Express each of the following numbers in exponential notation with correct significant figures:
- 704
- 0.03344
- 547.9
- 22086
- 1000.00
- 0.0000000651
- 0.007157
Check answers: [6]
- Indicate whether each of the following can be determined exactly or must be measured with some degree of uncertainty:
- the number of eggs in a basket
- the mass of a dozen eggs
- the number of gallons of gasoline necessary to fill an automobile gas tank
- the number of cm in 2 m
- the mass of a textbook
- the time required to drive from San Francisco to Kansas City at an average speed of 53 mi/h
- Indicate whether each of the following can be determined exactly or must be measured with some degree of uncertainty:
- the number of seconds in an hour
- the number of pages in this book
- the number of grams in your weight
- the number of grams in 3 kilograms
- the volume of water you drink in one day
- the distance from San Francisco to Kansas City
Check answers: [7]
- How many significant figures are contained in each of the following measurements?
- 38.7 g
- 2 × 1018 m
- 3,486,002 kg
- 9.74150 × 10−4 J
- 0.0613 cm3
- 17.0 kg
- 0.01400 g/mL
- How many significant figures are contained in each of the following measurements?
- 53 cm
- 2.05 × 108 m
- 86,002 J
- 9.740 × 104 m/s
- 10.0613 m3
- 0.17 g/mL
- 0.88400 s
Check answers: [8]
- The following quantities were reported on the labels of commercial products. Determine the number of significant figures in each.
- 0.0055 g active ingredients
- 12 tablets
- 3% hydrogen peroxide
- 5.5 ounces
- 473 mL
- 1.75% bismuth
- 0.001% phosphoric acid
- 99.80% inert ingredients
- Round off each of the following numbers to two significant figures:
- 0.436
- 9.000
- 27.2
- 135
- 1.497 × 10−3
- 0.445
Check answers: [9]
- Round off each of the following numbers to two significant figures:
- 517
- 86.3
- 6.382 × 103
- 5.0008
- 22.497
- 0.885
- Perform the following calculations and report each answer with the correct number of significant figures.
- 628 × 342
- (5.63 × 102) × (7.4 × 103)
- [latex]\frac{28.0}{13.483}[/latex]
- 8119 × 0.000023
- 14.98 + 27,340 + 84.7593
- 42.7 + 0.259
Check answers: [10]
- Perform the following calculations and report each answer with the correct number of significant figures.
- 62.8 × 34
- 0.147 + 0.0066 + 0.012
- 38 × 95 × 1.792
- 15 – 0.15 – 0.6155
- [latex]8.78 \times (\frac{0.0500}{0.478})[/latex]
- 140 + 7.68 + 0.014
- 28.7 – 0.0483
- [latex]\frac{(88.5-87.57)}{45.13}[/latex]
- Consider the results of the archery contest shown in this figure.
- Which archer is most precise?
- Which archer is most accurate?
- Who is both least precise and least accurate?
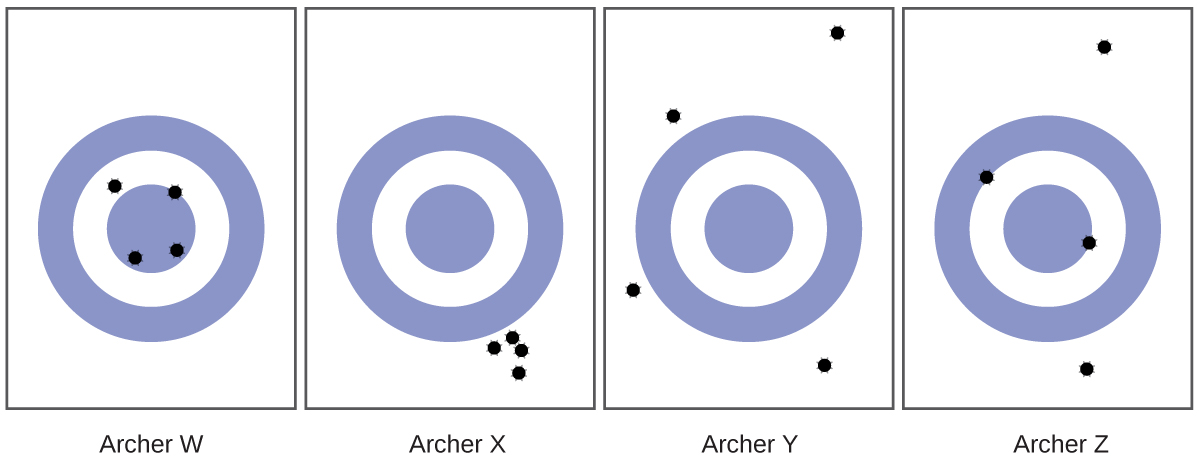
Check answers: [11]
- Classify the following sets of measurements as accurate, precise, both, or neither.
- Checking for consistency in the weight of chocolate chip cookies: 17.27 g, 13.05 g, 19.46 g, 16.92 g
- Testing the volume of a batch of 25-mL pipettes: 27.02 mL, 26.99 mL, 26.97 mL, 27.01 mL
- Determining the purity of gold: 99.9999%, 99.9998%, 99.9998%, 99.9999%
2.3 Mathematical Treatment of Measurement Results
- Write conversion factors (as ratios) for the number of:
- yards in 1 meter
- liters in 1 liquid quart
- pounds in 1 kilogram
Check answers: [12]
- Write conversion factors (as ratios) for the number of:
- kilometres in 1 mile
- litres in 1 cubic foot
- grams in 1 ounce
- The label on a soft drink bottle gives the volume in two units: 2.0 L and 67.6 fl oz. Use this information to derive a conversion factor between the English and metric units. How many significant figures can you justify in your conversion factor?
Check answers: [13] - The label on a box of cereal gives the mass of cereal in two units: 978 grams and 34.5 oz. Use this information to find a conversion factor between the English and metric units. How many significant figures can you justify in your conversion factor?
- Soccer is played with a round ball having a circumference between 27 and 28 in. and a weight between 14 and 16 oz. What are these specifications in units of centimetres and grams?
Check answers: [14] - A woman’s basketball has a circumference between 28.5 and 29.0 inches and a maximum weight of 20 ounces (two significant figures). What are these specifications in units of centimetres and grams?
- How many millilitres of a soft drink is contained in a 12.0-oz can?
Check answers: [15] - A barrel of oil is exactly 42 gal. How many litres of oil are in a barrel?
- The diameter of a red blood cell is about 3 × 10−4 in. What is its diameter in centimetres?
Check answers: [16] - The distance between the centres of the two oxygen atoms in an oxygen molecule is 1.21 × 10−8 cm. What is this distance in inches?
- Is a 197-lb weight lifter light enough to compete in a class limited to those weighing 90 kg or less?
Check answers: [17] - A very good 197-lb weight lifter lifted 192 kg in a move called the clean and jerk. What was the mass of the weight lifted in pounds?
- Many medical laboratory tests are run using 5.0 μL blood serum. What is this volume in millilitres?
Check answers: [18] - If an aspirin tablet contains 325 mg aspirin, how many grams of aspirin does it contain?
- Use scientific (exponential) notation to express the following quantities in terms of the SI base units in Table 2.1c in Chapter 2.1 Measurements:
- 0.13 g
- 232 Gg
- 5.23 pm
- 86.3 mg
- 37.6 cm
- 54 μm
- 1 Ts
- 27 ps
- 0.15 mK
Check answers: [19]
- Complete the following conversions between SI units.
- 612 g = ________ mg
- 8.160 m = ________ cm
- 3779 μg = ________ g
- 781 mL = ________ L
- 4.18 kg = ________ g
- 27.8 m = ________ km
- 0.13 mL = ________ L
- 1738 km = ________ m
- 1.9 Gg = ________ g
- Gasoline is sold by the litre in many countries. How many litres are required to fill a 12.0-gal gas tank?
Check answers: [20]
- Milk is sold by the litre in many countries. What is the volume of exactly 1/2 gal of milk in litres?
- A long ton is defined as exactly 2240 lb. What is this mass in kilograms?
Check answers: [21]
- Make the conversion indicated in each of the following:
- the men’s world record long jump, 29 ft 4¼ in., to meters
- the greatest depth of the ocean, about 6.5 mi, to kilometres
- the area of the state of Oregon, 96,981 mi2, to square kilometres
- the volume of 1 gill (exactly 4 oz) to millilitres
- the estimated volume of the oceans, 330,000,000 mi3, to cubic kilometres.
- the mass of a 3525-lb car to kilograms
- the mass of a 2.3-oz egg to grams
- Make the conversion indicated in each of the following:
- the length of a soccer field, 120 m (three significant figures), to feet
- the height of Mt. Kilimanjaro, at 19,565 ft the highest mountain in Africa, to kilometres
- the area of an 8.5 t 11-inch sheet of paper in cm2
- the displacement volume of an automobile engine, 161 in.3, to litres
- the estimated mass of the atmosphere, 5.6 t 1015 tons, to kilograms
- the mass of a bushel of rye, 32.0 lb, to kilograms
- the mass of a 5.00-grain aspirin tablet to milligrams (1 grain = 0.00229 oz)
Check answers: [22]
- Many chemistry conferences have held a 50-Trillion Angstrom Run (two significant figures). How long is this run in kilometres and in miles? (1 Å = 1 × 10−10 m)
- A chemist’s 50-Trillion Angstrom Run would be an archeologist’s 10,900 cubit run. How long is one cubit in meters and in feet? (1 Å = 1 × 10−8 cm)
Check answers: [23]
- The gas tank of a certain luxury automobile holds 22.3 gallons according to the owner’s manual. If the density of gasoline is 0.8206 g/mL, determine the mass in kilograms and pounds of the fuel in a full tank.
- As an instructor is preparing for an experiment, he requires 225 g phosphoric acid. The only container readily available is a 150-mL Erlenmeyer flask. Is it large enough to contain the acid, whose density is 1.83 g/mL?
Check answers: [24]
- To prepare for a laboratory period, a student lab assistant needs 125 g of a compound. A bottle containing 1/4 lb is available. Did the student have enough of the compound?
- A chemistry student is 159 cm tall and weighs 45.8 kg. What is her height in inches and weight in pounds?
Check answers: [25]
- In a recent Grand Prix, the winner completed the race with an average speed of 229.8 km/h. What was his speed in miles per hour, meters per second, and feet per second?
- Solve these problems about lumber dimensions.
- To describe to a European how houses are constructed in the US, the dimensions of “two-by-four” lumber must be converted into metric units. The thickness × width × length dimensions are 1.50 in. × 3.50 in. × 8.00 ft in the US. What are the dimensions in cm × cm × m?
- This lumber can be used as vertical studs, which are typically placed 16.0 in. apart. What is that distance in centimetres?
Check answers: [26]
- The mercury content of a stream was believed to be above the minimum considered safe—1 part per billion (ppb) by weight. An analysis indicated that the concentration was 0.68 parts per billion. What quantity of mercury in grams was present in 15.0 L of the water, the density of which is 0.998 g/ml? ([latex]1\;\text{ppb\;Hg} = \frac{\text{1 ng Hg}}{\text{1 g water}}[/latex])
- Calculate the density of aluminum if 27.6 cm3 has a mass of 74.6 g.
Check answers: [27]
- Osmium is one of the densest elements known. What is its density if 2.72 g has a volume of 0.121 cm3?
- Calculate these masses.
- What is the mass of 6.00 cm3 of mercury, density = 13.5939 g/cm3?
- What is the mass of 25.0 mL octane, density = 0.702 g/cm3?
Check answers: [28]
- Calculate these masses.
- What is the mass of 4.00 cm3 of sodium, density = 0.97 g/cm3 ?
- What is the mass of 125 mL gaseous chlorine, density = 3.16 g/L?
- Calculate these volumes.
- What is the volume of 25 g iodine, density = 4.93 g/cm3?
- What is the volume of 3.28 g gaseous hydrogen, density = 0.089 g/L?
Check answers: [29]
- Calculate these volumes.
- What is the volume of 11.3 g graphite, density = 2.25 g/cm3?
- What is the volume of 39.657 g bromine, density = 2.928 g/cm3?
- Convert the boiling temperature of gold, 2966 °C, into degrees Fahrenheit and kelvin.
Check answers: [30]
- Convert the temperature of scalding water, 54 °C, into degrees Fahrenheit and kelvin.
- Convert the temperature of the coldest area in a freezer, −10 °F, to degrees Celsius and kelvin.
Check answers: [31]
- Convert the temperature of dry ice, −77 °C, into degrees Fahrenheit and kelvin.
- Convert the boiling temperature of liquid ammonia, −28.1 °F, into degrees Celsius and kelvin.
Check answers: [32]
- The label on a pressurized can of spray disinfectant warns against heating the can above 130 °F. What are the corresponding temperatures on the Celsius and kelvin temperature scales?
- The weather in Europe was unusually warm during the summer of 1995. The TV news reported temperatures as high as 45 °C. What was the temperature on the Fahrenheit scale?
Check answers: [33]
Attribution & References
Except where otherwise noted, this page is adapted by JR van Haarlem from “”1.4 Measurements“, “1.5 Measurement Uncertainty, Accuracy, and Precision” and “1.6 Mathematical Treatment of Measurement Results“” In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax)
- about a yard ↵
- (a) kilograms; (b) meters; (c) meters/second; (d) kilograms/cubic meter; (e) kelvin; (f) square meters; (g) cubic meters ↵
- (a) centi-, X 10−2; (b) deci-, X 10−1; (c) Giga-, X 109; (d) kilo-, X 103; (e) milli-, X 10−3; (f) nano-, X 10−9; (g) pico-, X 10−12; (h) tera-, X 1012 ↵
- (a) m = 18.58 g, V = 5.7 mL. (b) d = 3.3 g/mL (c) dioptase (copper cyclosilicate, d = 3.28—3.31 g/mL); malachite (basic copper carbonate, d = 3.25—4.10 g/mL); Paraiba tourmaline (sodium lithium boron silicate with copper, d = 2.82—3.32 g/mL) ↵
- (a) Vwithout block= 25.5 mL, Vwith block=28.3 mL, Vdisplaced=2.8 mL (b) mwater = d x V = 2.8 g/mL (c) mfoam block=2.76 g (same as mass of water displaced) ↵
- (a) 7.04 × 10^2; (b) 3.344 × 10^−2; (c) 5.479 × 10^2; (d) 2.2086 × 10^4; (e) 1.00000 × 10^3; (f) 6.51 × 10^−8; (g) 7.157 × 10^−3 ↵
- (a) exact; (b) exact; (c) uncertain; (d) exact; (e) uncertain; (f) uncertain ↵
- (a) two; (b) three; (c) five; (d) four; (e) six; (f) two; (g) five ↵
- (a) 0.44; (b) 9.0; (c) 27; (d) 140; (e) 1.5 × 10^−3; (f) 0.44 ↵
- (a) 2.15 × 10^5; (b) 4.2 × 10^6; (c) 2.08; (d) 0.19; (e) 27,440; (f) 43.0 ↵
- (a) Archer X; (b) Archer W; (c) Archer Y ↵
- (a) 1.0936 yd m-1 ; (b) 0.94635 L qt-1 ; (c) 2.2046 lb kg-1 ↵
- 2.0 L (67.6 fl oz)-1=0.030 L (1 fl oz)-1 ↵
- 68–71 cm; 400–450 g ↵
- 355 mL ↵
- 8 × 10−4 cm ↵
- yes; weight = 89.4 kg ↵
- 5.0 × 10−3 mL ↵
- (a) 1.3 × 10−4 kg; (b) 2.32 × 108 kg; (c) 5.23 × 10−12 m; (d) 8.63 × 10−5 kg; (e) 3.76 × 10−1 m; (f) 5.4 × 10−5 m; (g) 1 × 1012 s; (h) 2.7 × 10−11 s; (i) 1.5 × 10−4 K ↵
- 45.4 L ↵
- 1.0160 × 103 kg ↵
- (a) 394 ft; (b) 5.9634 km; (c) 6.0 × 102; (d) 2.64 L; (e) 5.1 × 1018 kg; (f) 14.5 kg; (g) 324 mg ↵
- 0.46 m; 1.5 ft/cubit ↵
- Yes, the acid’s volume is 123 mL. ↵
- 62.6 in (about 5 ft 3 in.) and 101 lb. ↵
- (a) 3.81 cm × 8.89 cm × 2.44 m; (b) 40.6 cm ↵
- 2.70 g/cm3 ↵
- (a) 81.6 g; (b) 17.6 g ↵
- (a) 5.1 mL; (b) 37 L ↵
- 5371 °F, 3239 K ↵
- −23 °C, 250 K ↵
- −33.4 °C, 239.8 K ↵
- 113 °F ↵

