3.3 Compounds and Formulas
Learning Objectives
By the end of this section, you will be able to:
- Define molecular and structural formulas
- Define ionic and molecular (covalent) compounds
- Determine the number of atoms of each element in a compound
Scientists in Action: Dr. Uma Chowdhry
Dr. Uma Chowdhry is a retired senior vice president and chief science and technology officer for DuPont, where she worked from 1977 to 2010. She was born in Mumbai, then came to the U.S. for her Masters Degree from Caltech and her PhD in material science from MIT.
Dr. Chowdhry started as a research scientist for Dupont and, throughout her career, she was responsible for overseeing a large number of projects. In 1987 the research efforts she led in ceramic superconducting materials generated over 20 patents and 50 publications.
There is a very good chance you own something that relies on materials and technology developed by DuPont during Dr. Chowdhry’s time.
See Dr. Chowdhry talking more about her life in Women in Chemistry: Uma Chowdhry [New Tab].
Chemical Formulae
A molecular formula is a representation of a molecule that uses chemical symbols to indicate the types of atoms followed by subscripts to show the number of atoms of each type in the molecule. (A subscript is used only when more than one atom of a given type is present.) Molecular formulas are also used as abbreviations for the names of compounds.
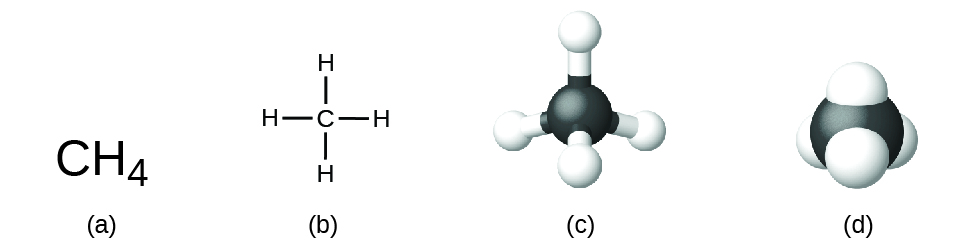
The structural formula for a compound gives the same information as its molecular formula (the types and numbers of atoms in the molecule) but also shows how the atoms are connected in the molecule. The structural formula for methane contains symbols for one C atom and four H atoms, indicating the number of atoms in the molecule (Figure 3.3b). The lines represent bonds that hold the atoms together. (A chemical bond is an attraction between atoms or ions that holds them together in a molecule or a crystal.) We will discuss chemical bonds and see how to predict the arrangement of atoms in a molecule later. For structural formula now, simply know that the lines are an indication of how the atoms are connected in a molecule. A ball-and-stick model shows the geometric arrangement of the atoms with atomic sizes not to scale, and a space-filling model shows the relative sizes of the atoms.
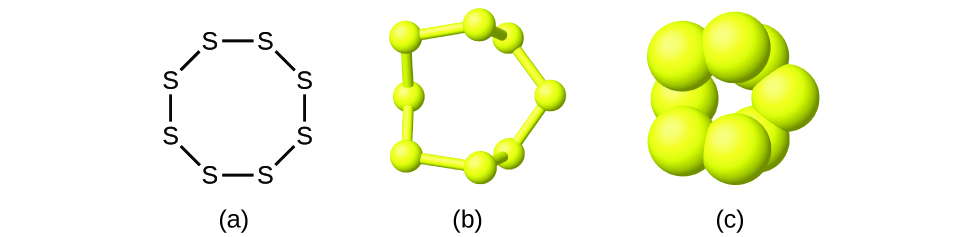
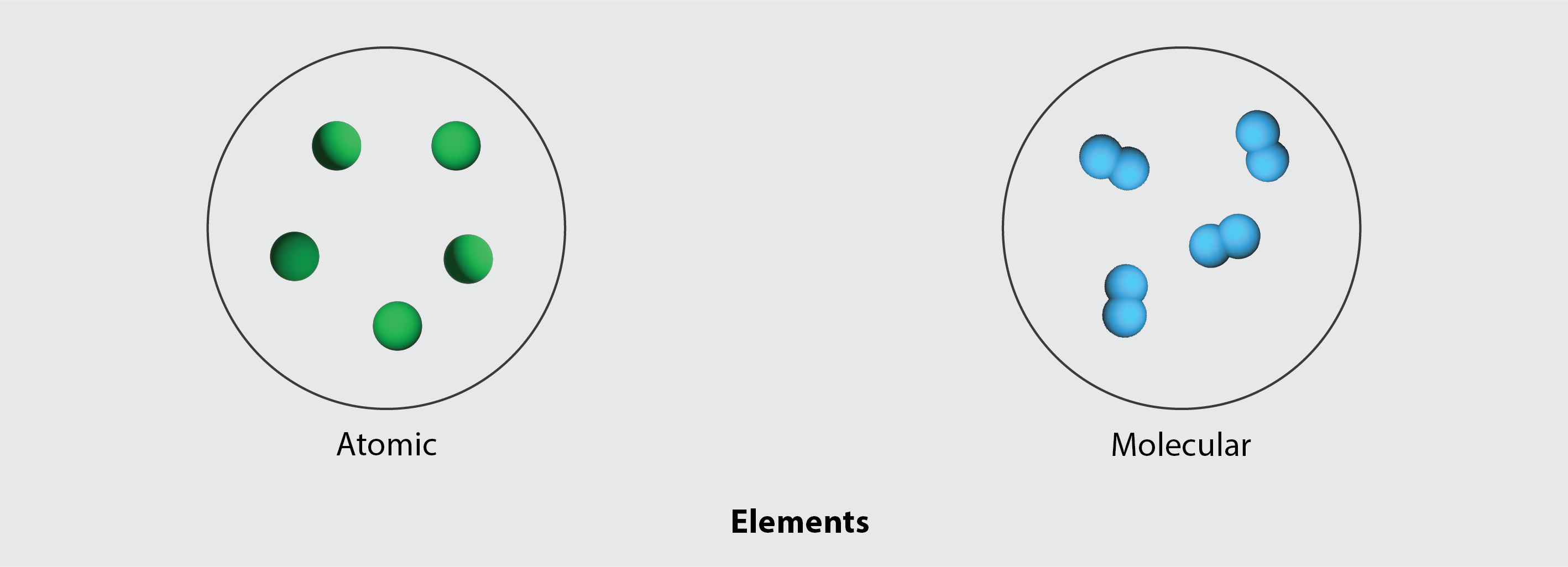
Although many elements consist of discrete, individual atoms, some exist as molecules made up of two or more atoms of the element chemically bonded together. For example, most samples of the elements hydrogen, oxygen, and nitrogen are composed of molecules that contain two atoms each (called diatomic molecules) and thus have the molecular formulas H2, O2, and N2, respectively. Other elements commonly found as diatomic molecules are fluorine (F2), chlorine (Cl2), bromine (Br2), and iodine (I2). The most common form of the element sulfur is composed of molecules that consist of eight atoms of sulfur; its molecular formula is S8 (Figure 3.3c).
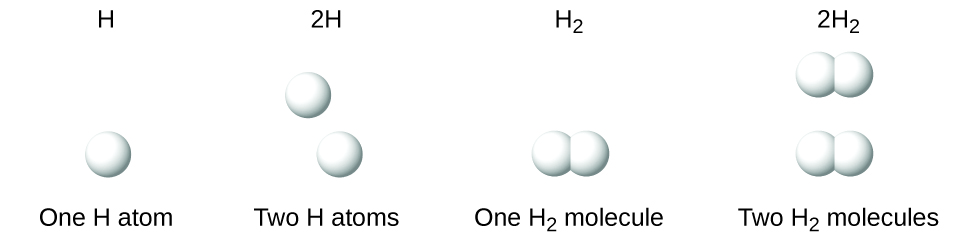
It is important to note that a subscript following a symbol and a number in front of a symbol do not represent the same thing; for example, H2 and 2H represent distinctly different species. H2 is a molecular formula; it represents a diatomic molecule of hydrogen, consisting of two atoms of the element that are chemically bonded together. The expression 2H, on the other hand, indicates two separate hydrogen atoms that are not combined as a unit. The expression 2H2 represents two molecules of diatomic hydrogen (Figure 3.3d).
Compounds are formed when two or more elements chemically combine, resulting in the formation of bonds. For example, hydrogen and oxygen can react to form water, and sodium and chlorine can react to form table salt.
As discussed previously, we can describe a compound with a molecular formula, in which the subscripts indicate the actual numbers of atoms of each element in a molecule of the compound. For example, it can be determined experimentally that benzene contains two elements, carbon (C) and hydrogen (H), and that for every carbon atom in benzene, there is one hydrogen atom. An experimental determination of the molecular mass reveals that a molecule of benzene contains six carbon atoms and six hydrogen atoms, so the molecular formula for benzene is C6H6 (Figure 3.3e).
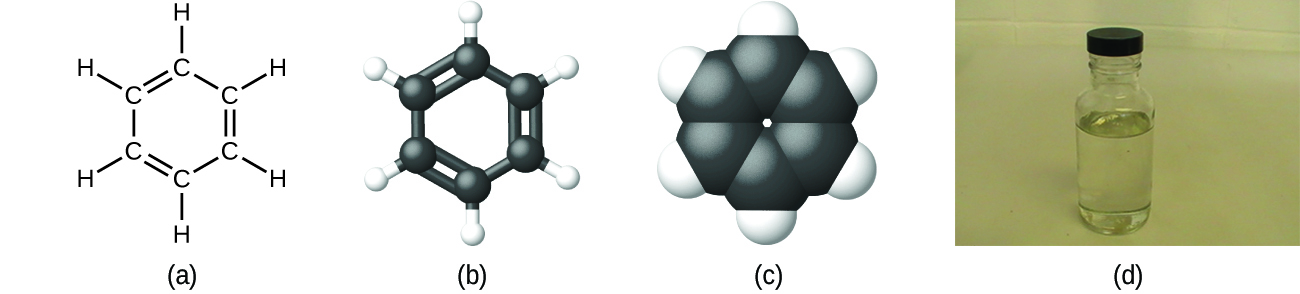
Since elements on the periodic table can exist naturally as single atom or as diatomic molecules, they can therefore be classified as being either atomic, or molecular, as shown in Figure 3.3f.
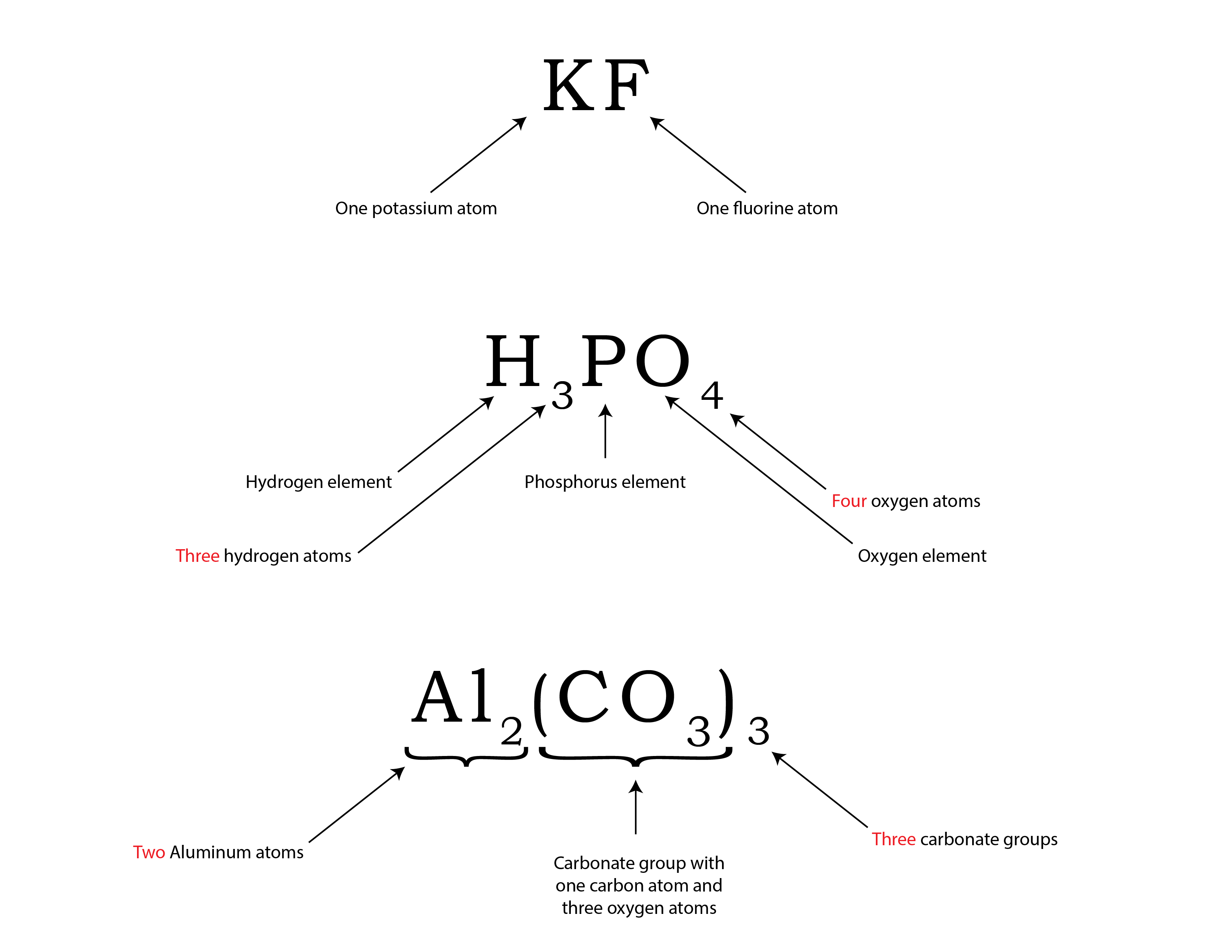
When writing chemical formulae, we represent each atom in the molecule with its symbol from the periodic table, followed by a subscript that represents the number of atoms of that element in the molecule. If the element in the molecule in question contains only one single atom, the number “1” is not used as a subscript as a general rule. Some examples are shown in Figure 3.3g.
Example 3.3a
Molecular Formulas
Molecules of glucose (blood sugar) contain 6 carbon atoms, 12 hydrogen atoms, and 6 oxygen atoms. What is the molecular formula of glucose?
Solution
The molecular formula is C6H12O6 because one molecule actually contains 6 C, 12 H, and 6 O atoms.
Exercise 3.3a
A molecule of metaldehyde (a pesticide used for snails and slugs) contains 8 carbon atoms, 16 hydrogen atoms, and 4 oxygen atoms. What is the molecular formula of metaldehyde?
Check Your Answer[1]
It is important to be aware that it may be possible for the same atoms to be arranged in different ways: Compounds with the same molecular formula may have different atom-to-atom bonding and therefore different structures.
Example 3.3b
Determine the number of atoms of each element and the total number of atoms for the compound AlCl3 ?
Solution
1 Al atom
3 Cl atoms
Total number of atoms in AlCl3 is 4 atoms since 1 Al atom + 3 Cl atoms = 4 total atoms
Source: “Example 3.3b” by Adrienne Richards is licensed under CC BY NC 4.0.
Example 3.3c
How many atoms of each element and the total number of atoms are present in the compound CaCO3?
Answer
1 Ca
1 C
3 O
The total number of atoms in CaCO3 is 5 since 1 Ca + 1 C + 3 O = 5 atoms.
Source: “Example 3.3c” by Adrienne Richards is licensed under CC BY NC 4.0.
The Formation of Chemical Compounds
In ordinary chemical reactions, the nucleus of each atom (and thus the identity of the element) remains unchanged. Electrons, however, can be added to atoms by transfer from other atoms, lost by transfer to other atoms, or shared with other atoms. The transfer and sharing of electrons among atoms govern the chemistry of the elements. During the formation of some compounds, atoms gain or lose electrons, and form electrically charged particles called ions (Figure 3.3h).
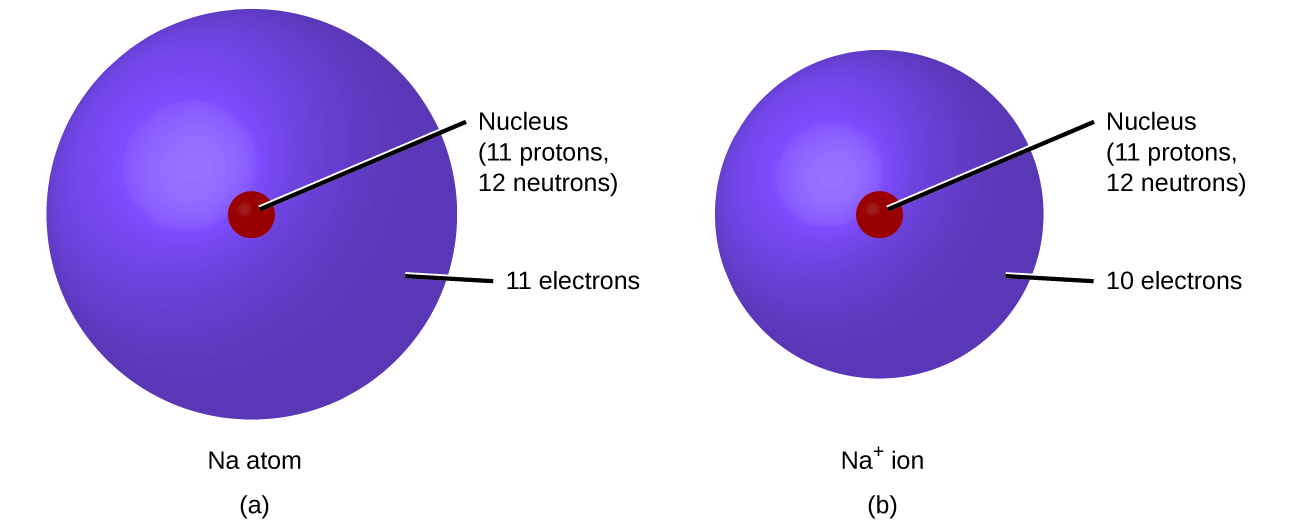
The nature of the attractive forces that hold atoms or ions together within a compound is the basis for classifying chemical bonding. When electrons are transferred and ions form, ionic bonds result. Ionic bonds are electrostatic forces of attraction, that is, the attractive forces experienced between objects of opposite electrical charge (in this case, cations and anions). When electrons are “shared” and molecules form, covalent bonds result. Covalent bonds are the attractive forces between the positively charged nuclei of the bonded atoms and one or more pairs of electrons that are located between the atoms. Compounds are classified as ionic or molecular (covalent) on the basis of the bonds present in them (Figure 3.3i).
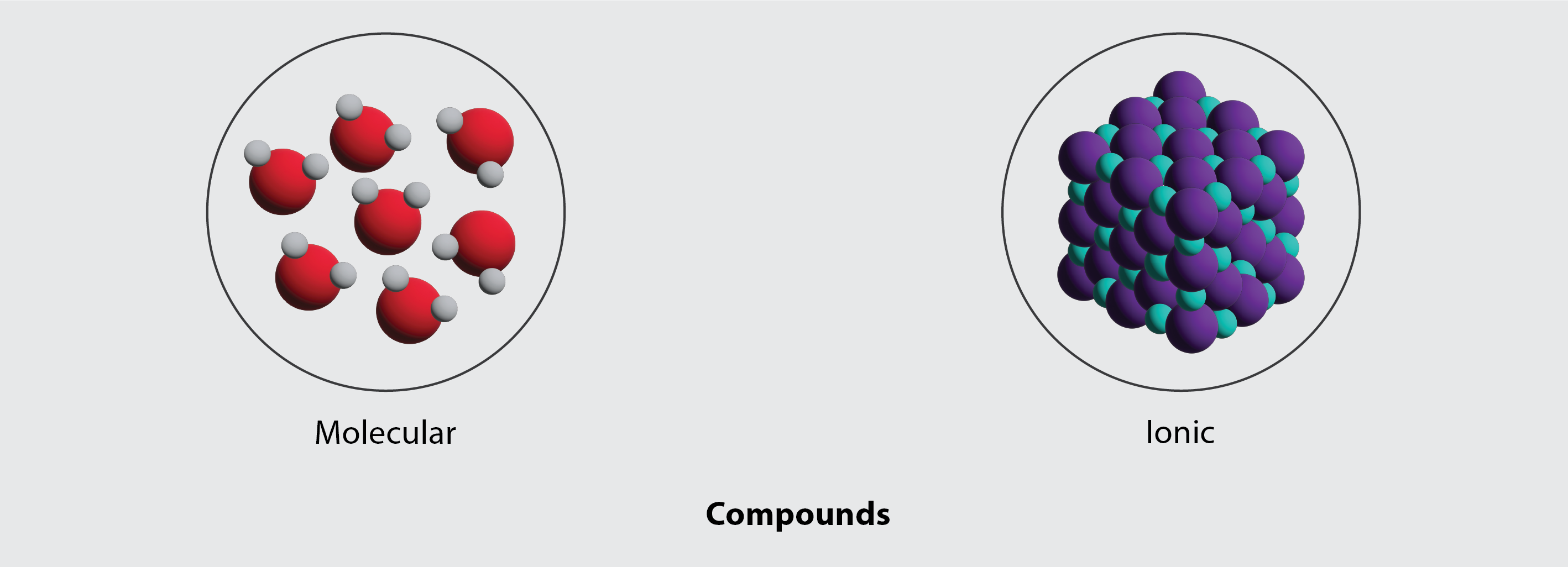
Ionic Compounds
When an element composed of atoms that readily lose electrons (a metal) reacts with an element composed of atoms that readily gain electrons (a nonmetal), a transfer of electrons usually occurs, producing ions. A compound that contains ions and is held together by ionic bonds is called an ionic compound. The periodic table can help us recognize many of the compounds that are ionic: When a metal is combined with one or more nonmetals, the compound is usually ionic.
Watch Conductivity in molten salt (1 mins)
Video source: Ashkenazi, G. (2012, November 15). Conductivity molten salt [Video]. YouTube.
In every ionic compound, the total number of positive charges of the cations equals the total number of negative charges of the anions. Thus, ionic compounds are electrically neutral overall, even though they contain positive and negative ions. We can use this observation to help us write the formula of an ionic compound. The formula of an ionic compound must have a ratio of ions such that the numbers of positive and negative charges are equal.
Molecular Compounds
Many compounds do not contain ions but instead consist solely of discrete, neutral molecules. These molecular compounds (covalent compounds) result when atoms share, rather than transfer (gain or lose), electrons. Covalent bonding is an important and extensive concept in chemistry, and it will be treated in considerable detail in a later chapter of this text. We can often identify molecular compounds on the basis of their physical properties. Under normal conditions, molecular compounds often exist as gases, low-boiling liquids, and low-melting solids, although many important exceptions exist.
Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination of nonmetals.
Links to Interactive Learning Tools
Practice Chemical Formulas and Atom Counting by the Physics Classroom.
Attribution & References
Except where otherwise noted, this section is adapted by Adrienne Richards from:
- “2.5 Chemical Formulas” In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax) AND
- “2.6 Ionic and Molecular Compounds” In Chemistry 2e (OpenStax) by Paul Flowers, Klaus Theopold, Richard Langley, & William R. Robinson, licensed under CC BY 4.0. / Adaptations include combining sections 2.4 and 2.6. Access for free at Chemistry 2e (OpenStax).
- Molecular formula, C8H16O4 ↵
chemical equation in which all reactants and products are represented as neutral substances
shows the atoms in a molecule and how they are connected
molecules that contain two identical atoms chemically bonded together
pure substance that can be decomposed into two or more elements
electrically charged atom or molecule (contains unequal numbers of protons and electrons)
electrostatic forces of attraction between the oppositely charged ions of an ionic compound
positively charged atom or molecule (contains fewer electrons than protons)
negatively charged atom or molecule (contains more electrons than protons)
attractive force between the nuclei of a molecule’s atoms and pairs of electrons between the atoms
compound composed of cations and anions combined in ratios, yielding an electrically neutral substance
(also, covalent compound) composed of molecules formed by atoms of two or more different elements
(also, molecular compound) composed of molecules formed by atoms of two or more different elements

