5.2 Electric Charge
Learning Objectives
By the end of this section, you will be able to:
- Describe the concept of electric charge and its properties
In the two centuries since Dalton developed his ideas, scientists have made significant progress in advancing our understanding of atomic theory. Some of this development came from the results of several pioneering experiments that revealed details of electric charge and discovery of ions. Before you learn about the internal structure of an atom and the experiments that led to their discovery, it is important to outline key concepts in electric charge and about the discovery of ions.
Electric Charge
You are certainly familiar with electronic devices that you activate with the click of a switch, from computers to cell phones to television. And you have certainly seen electricity in a flash of lightning during a heavy thunderstorm. But you have also most likely experienced electrical effects in other ways, maybe without realizing that an electric force was involved. Let’s take a look at some of these activities and see what we can learn from them about electric charges and forces.
Discoveries
You have probably experienced the phenomenon of static electricity: When you first take clothes out of a dryer, many (not all) of them tend to stick together; for some fabrics, they can be very difficult to separate. Another example occurs if you take a woolen sweater off quickly—you can feel (and hear) the static electricity pulling on your clothes, and perhaps even your hair. If you comb your hair on a dry day and then put the comb close to a thin stream of water coming out of a faucet, you will find that the water stream bends toward (is attracted to) the comb (Figure 5.2a).


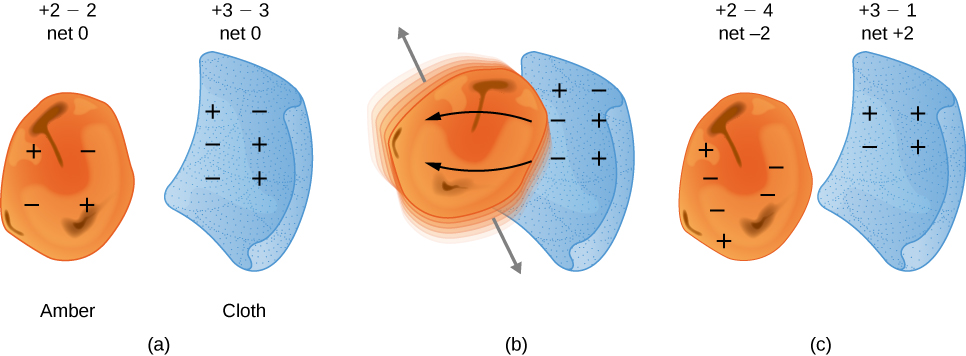
This suggested there were two types of an electric property, which eventually came to be called electric charge. It was concluded there were two types of electric charge – positive and negative. The difference between the two types of electric charge is in the directions of the electric forces that each type of charge causes:
- These forces are repulsive when the same type of charge exists on two interacting objects
- These forces are attractive when the charges are of opposite types
The most peculiar aspect of this new force is that it does not require physical contact between the two objects in order to cause an acceleration. This is an example of a so-called “long-range” force, (or, as James Clerk Maxwell later phrased it, “action at a distance”), which later became known as a form of induction.
The properties of electric charge are as follows:
- Charges can be positive and negative
- Electric force can be either attractive or repulsive
- If two interacting objects carry the same sign of charge, the force is repulsive.
- This interaction is referred to as electrostatic repulsion
- If the charges are of opposite sign, the force is attractive.
- This interaction is referred to as electrostatic attraction
- If two interacting objects carry the same sign of charge, the force is repulsive.
- The magnitude of the force decreases (rapidly) with increasing separation distance between objects. The magnitude of the force increases (rapidly) with decreasing separation distance between the objects.
- The force acts by contact or induction (without physical contact between the two objects)
- Not all objects are affected by this force
Exercise 5.2a
Check Your Learning Exercise (Text Version)
Read the following statement about electric charge and determine whether the statement is True OR False.
- Charges can be positive and neutral
- Electric force can be either attractive or equal
- If two interacting objects carry the same sign of charge, it results in electrostatic repulsion
- If the charges are of opposite sign, the force is attractive.
- If a balloon is rubbed on hair to gain charge and then is placed against a wall and sticks to the wall, the two objects have opposite charges
- The magnitude of the force decreases (rapidly) with decreasing separation distance between objects
- When two objects of similar charge repel each other without contact it is called induction.
- All objects are affected by electric force
Check Your Answer[1]
Source: “Exercise 5.2a” by Jackie MacDonald, licensed under CC BY-NC-SA 4.0
The discovery that matter (and its atoms) has properties of electric charge and contain both positive and negative charges led to the theory that a given neutral atom may be able to lose or gain such charges and become positively or negatively charged atoms, respectively. These charged atoms were later defined as positive ions – cations and negative ions – anions. This concept will be discussed in more detail in upcoming sections and chapters.
Attribution & References
Except where otherwise noted, this page is adapted by Jackie MacDonald from “Electric charge” In University Physics Volume 2 (Open Stax) by Samuel J. Ling, William Moebs, Jeff Sanny is licensed under CC BY 4.0. Access for free at University Physics Volume 2 (OpenStax)
-
For the following answers, any false answers, have been rewritten to show the correct statement. The bolded words (also noted with an *) were changed from the original false statement to make the statement true.
- False - Charges can be positive and *negative;
- False - Electric force can be either attractive or *repulsive;
- True;
- True;
- True;
- False - The magnitude of the force decreases (rapidly) with *increasing separation distance between objects OR The magnitude of the force *increases (rapidly) with decreasing separation distance between objects;
- True;
- False – *Not all objects are affected by electric force
buildup of electric charge on the surface of an object; the arrangement of the charge remains constant (“static”)
physical property of an object that causes it to be attracted toward or repelled from another charged object; each charged object generates and is influenced by a force called an electric force
noncontact force observed between electrically charged objects
phenomenon of two objects with like charges repelling each other
phenomenon of two objects with opposite charges attracting each other

