5.4 Defining the Nuclear Atom
Learning Objectives
By the end of this section, you will be able to:
- Summarize the structural characteristics of the nuclear atom
- Illustrate a simple model of the nuclear atom; locate its subatomic particles and their charges
- Explain why atoms have no overall charge
- Define ion and use the number of electrons lost or gained from an atom to calculate the overall charge of the ion and write the corresponding ion symbol.
The idea that matter is composed of tiny particles called atoms is at least 25 centuries old. It took until the twentieth century, however, for scientists to invent instruments that permitted them to probe inside an atom and find that it is not, as had been thought, hard and indivisible as Dalton theorized. Instead, experiments by Thomson, Rutherford, Chadwick, and other scientists revealed the atom is a complex structure composed of still smaller subatomic particles – electrons, protons, and neutrons.
Probing the Nuclear Atom
You have learned in the previous section that the first of these smaller particles were discovered by British physicist James (J. J.) Thomson in 1897. Named the electron, this particle is negatively charged. (It is the flow of these particles that produces currents of electricity, whether in lightning bolts or in the wires leading to your lamp.) Because an atom in its normal state is electrically neutral, each electron in an atom must be balanced by the same amount of positive charge.
The next step was to determine where in the atom the positive and negative charges were located. British physicist Ernest Rutherford devised the alpha-particle scattering, gold foil experiment that provided part of the answer to this question. The only way to account for the alpha particles that reversed direction when they hit the gold foil was to assume that nearly all of the mass, as well as all of the positive charge in each individual gold atom, is concentrated in a tiny centre or nucleus. When a positively charged alpha particle strikes a nucleus, it reverses direction, much as a cue ball reverses direction when it strikes another billiard ball. He termed this positive charge – a proton. Rutherford’s model also placed the other type of charge—the negative electrons—in orbit around this nucleus.
Rutherford’s model required that the electrons be in motion. Positive and negative charges attract each other, so stationary electrons would fall into the positive nucleus. Also, because both the electrons and the nucleus are extremely small, most of the atom is empty, which is why nearly all of Rutherford’s particles were able to pass right through the gold foil without colliding with anything. Rutherford’s model was a very successful explanation of the experiments he conducted, although eventually, scientists would discover that even the nucleus itself has structure.
Chadwick identified the neutron (n0). It has neither a positive nor a negative charge, so it is considered neutral. It was determined that neutrons are also located in the nucleus (centre) of the atom with protons, and like protons, have a similar mass.
Collectively, these experimental observations, especially Rutherford’s experiments, provided insight into the structure of the nuclear atom. The majority of the atom’s structure is made up of empty space, with a centrally located, very concentrated nucleus. The nucleus contains positively charged protons and neutrally charged neutrons. Combined, these account for the majority of the mass in a given atom. The negative electrons, which contribute very little to the overall mass of the atom, are in orbit around the nucleus within the empty space.
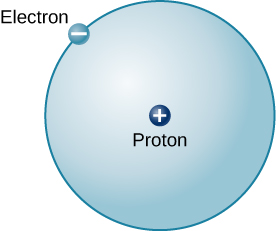
The simplest possible atom (and the most common one in the sun and stars) is the element hydrogen (H). The nucleus of ordinary hydrogen contains a single proton. Moving around this proton is a single electron. Recall, the mass of an electron is nearly 2000 times smaller than the mass of a proton, and the electron carries an amount of charge exactly equal to that of the proton but opposite in sign (Figure 5.4a). Opposite charges attract each other, so it is an electromagnetic force that holds the proton and electron together. But what about the neutron(s)? The diagram below does not show hydrogen with any neutrons. In fact, hydrogen (the first element on the periodic table) has only one proton in the nucleus and one orbiting electron but does not contain any neutrons. Hydrogen actually has 3 naturally occurring forms that are all a little bit different from one another. The one shown below is the most abundant form of hydrogen in nature. These different forms of the same element are called isotopes. The concept of isotopes will be discussed in this section and in greater detail later in this chapter.
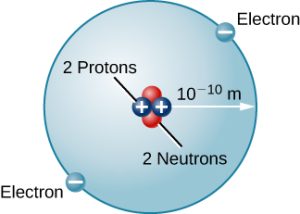
There are many other types of atoms in nature. Helium, for example, is the second-most abundant element in the Sun. Helium has two protons in its nucleus instead of the single proton that characterizes hydrogen. In addition, the helium nucleus contains two neutrons, particles with a mass comparable to that of the proton but with no electric charge. Moving around this nucleus are two electrons, so the total net charge of the helium atom is also zero (Figure 5.4b).
From this description of hydrogen and helium, perhaps you have guessed the pattern for building up all the elements (different types of atoms) that we find in the universe. The type of element is determined by the number of protons in the nucleus of the atom. Every element has a specific atomic number that equals the number of protons it contains; an element’s atomic number can be sourced from the periodic table. For example, any atom with six protons is the element carbon, with eight protons is oxygen, with 26 is iron, and with 92 is uranium. On Earth, a typical atom has the same number of electrons as protons, and these electrons follow complex orbital patterns around the nucleus (which will be covered in more detail in chapter 10 – modern atomic theory).
Next, another model of the nuclear atom is shown in Figure 5.4c.
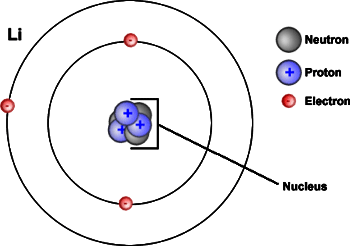
Formation of Ions
The number of protons, neutrons, and electrons an atom contains differentiates one type of atom from the next. In any given atom of an element when the number of positively charged protons and the number of negatively charged electrons are the same, the atom is neutral. Interestingly, an atom can lose or gain electrons – this is what gives an element its chemical properties, including its ability to combine with other elements to form new matter. When an atom has more or less electrons than protons, it is electrically charged and is called an ion. Recall that the relative charge of one proton is +1 and one electron is -1. The amount of charge is dependent on the number of electrons lost or gained. Ions are written using the atomic symbol with its quantity of charge and charge sign (+ or -) in superscript. Note for any atoms having a +1 or -1 charge, the 1 is omitted when writing the ion symbol.
The charge of an atom is determined as follows:
Atomic charge = number of protons − number of electrons
As will be discussed in more detail later in this book, atoms (and molecules) typically acquire charge by gaining or losing electrons. An atom that gains one or more electrons will exhibit a negative charge and is called an anion. Positively charged atoms called cations are formed when an atom loses one or more electrons. For example, a neutral sodium atom (atomic number = 11) has 11 protons and 11 electrons. If this atom loses one electron, it will become a cation with a 1+ charge (11 − 10 = 1+); its ion symbol is Na+. A neutral oxygen atom (atomic number = 8) has eight protons and 8 electrons, and if it gains two electrons it will become an anion with a 2− charge (8 − 10 = 2−); its ion symbol is O2-.
In summary (also refer to Table 5.4a):
- Atoms that lose electrons will have less electrons than protons and form positive ions called cations.
- Atoms that gain electrons will have more electrons than protons and form negative ions called anions.
| Starting Atom | Gain / Lose Electrons | More / Less Electrons than Protons | Type of Ion | Ion Classification | Determining Charge |
|---|---|---|---|---|---|
| Neutral Atom | loses electron(s) | LESS e–s | forms positive ion | cation | = # electrons lost to form ion |
| Neutral Atom | gains electron(s) | MORE e–s | forms negative ion | anion | = # electrons gained to form ion |
Source: “Table 5.4a” by Jackie MacDonald is licensed under CC BY-NC-SA 4.0.
Example 5.4a
Determining Ionic Symbols by Calculating Ionic Charge
A neutral lithium atom has 3 electrons and 3 protons. Determine the electric charge and write the ionic symbol for a lithium cation containing 2 electrons.
Solution
Lithium forms a CATION, a positive ion: The lithium ion has two electrons (2 e–) and three protons (3 p+), which means the neutral atom lost one electron (1 e–).
CHARGE of Li ion = protons – electrons = 3 – 2 = +1; therefore, the ion has a net charge of +1.
ION SYMBOL for the lithium ion is Li+
Example 5.4b
Determining Ionic Symbols by Calculating Ionic Charge
A neutral sulfur atom has 16 electrons and 16 protons. Determine the electric charge and write the ionic symbol for an sulfur anion containing 18 electrons.
Solution
Sulfur forms an ANION, a negative ion: The Sulfur ion has 18 electrons (18 e–) and 16 protons (16 p+), which means the neutral atom gained two electron (2 e–).
CHARGE of S ion = protons – electrons = 16 – 18 = -2; therefore, the ion has an net charge of -2.
ION SYMBOL for the sulfur ion is S2-
Example 5.4c
Determining Ionic Symbols by Calculating Ionic Charge
A neutral chlorine atom has 17 protons and 17 electrons. It gains one electron to form a chloride ion.
- Determine the electric charge on the ion and write the ionic symbol.
- Is the ion considered a cation or anion?
- How many total electrons does the chloride ion have?
Solution
A neutral chlorine atom gains one electron, so it will have a 1- charge; its symbol is Cl–. Chlorine formed a negative ion, and is classified as an anion. Chloride ion has 18 electrons total (17 e– + 1 e– = 18 e–)
Exercise 5.4a
Check Your Learning Exercise (Text Version)
From the options provided in brackets for each statement, decide which of the provided choices is correct.
- Arsenic as a neutral atom contains 33 protons and (32 / 33 / 36) electrons. As an ion it can have 36 electrons. The charge on this ion would be (3+ / 3–). This type of ion is a(n) (cation / anion). The ion symbol for the ion is (As3+ / As3-).
- Barium as a neutral atom contains 56 protons and (54 / 56 / 58) electrons. As an ion it has 54 electrons. The charge on this ion would be (2+ / 1+ / 1– / 2–). This type of ion is a(n) (cation / anion). The ion symbol for the ion is (Ba2+ / Ba2- ).
- Potassium as a neutral atom contains 19 protons and (18 / 19 / 20) electrons. Potassium loses one electron to form a(n) (cation / anion) that has a (1– / 1+) charge. This ion will have total of (18 / 19 / 20) electrons. The ion symbol for the ion is (K+ / P+ / K– / P–).
Check Your Answer[1]
Source: “Exercise 5.4a” by Jackie MacDonald is licensed under CC BY-NC-SA 4.0.
You will learn more about elements forming ions when reviewing concepts in chemical nomenclature, chemical reactions, and chemical bonding.
The Atomic Nucleus
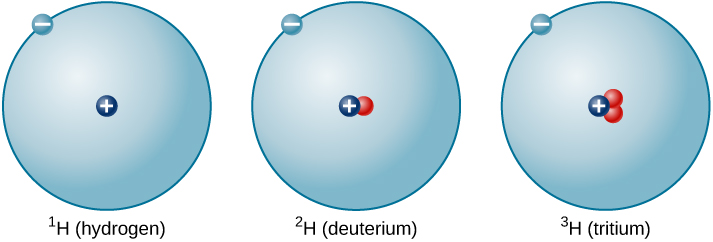
The ratio of neutrons to protons increases as the number of protons increases, but each element is unique. The number of neutrons is not necessarily the same for all atoms of a given element. As aforementioned, most hydrogen atoms contain no neutrons at all. There are, however, hydrogen atoms that contain one proton and one neutron, and others that contain one proton and two neutrons. The various types of hydrogen nuclei with different numbers of neutrons are called isotopes of hydrogen (Figure 5.4d), and all other elements have isotopes as well. You can think of isotopes as siblings in the same element “family”—closely related but with different characteristics and behaviours. Turns out the element Hydrogen has three different isotopic forms, all containing one proton and one electron: Hydrogen – 1; Hydrogen – 2; and Hydrogen – 3, having 0, 1, and 2 neutrons in their atom nucleus, respectively. Hydrogen – 1 is the most abundant of the hydrogen isotopes and accounts for 99.9885% of all Hydrogen found in nature.
Exercise 5.4b
Check Your Learning Exercise (Text Version)
Review the word list provided and choose a word or phase in the (BLANK) to make the statements correct. Each word will be used once.
WORD LIST: (11 words/phrases)
atomic number, protons, positively, electrons, neutron, isotopes, mass, empty space, ions, cations, anions
- The nuclear model of the atom is made up of mostly (BLANK).
- The atomic number tells you how many (BLANK) (and electrons) there are in a neutral atom.
- The whole number found on the periodic table near an element symbol is called the (BLANK). It is unique for each element.
- An atom with the same number of protons but different number of neutrons are called (BLANK).
- The subatomic particle that has no charge is called a (BLANK).
- The nucleus of the atom is (BLANK) charged. It contains protons and neutrons, which are the main subatomic particles that contribute to the overall (BLANK) of the atom.
- Atoms can gain or lose (BLANK) to form positive or negative (BLANK).
- A positive ion is called a(n) (BLANK); a negative ion is called a(n) (BLANK).
Check Your Answer[2]
Source: “Exercise 5.4b” by Jackie MacDonald is licensed under CC BY-NC-SA 4.0.
Exercise 5.4c
Practice using the following PhET simulation: Build an Atom
Links to Interactive Learning Tools
Explore Subatomic Particles from the Physics Classroom.
Explore Subatomic Particle Terminology from eCampusOntario H5P Studio.
Attribution & References
- (1) Arsenic as a neutral atom contains 33 protons and 33 electrons. As an ion it can have 36 electrons. The charge on this ion would be 3-. This type of ion is a(n) anion. The ion symbol for the ion is As3-. (2) Barium as a neutral atom contains 56 protons and 56 electrons. As an ion it has 50 electrons. The charge on this ion would be 2+. This type of ion is cation. The ion symbol for the ion is Ba2+. (3) Potassium as a neutral atom contains 19 protons and 19 electrons. Potassium loses one electron to form cation that has a 1+ charge. This ion will have total of 18 electrons. The ion symbol for the ion is K+. ↵
- (1) empty space; (2) protons; (3) atomic number; (4) isotopes; (5) neutron; (6) positively, mass; (7) electrons, ions; (8) cation, anion ↵
atoms that contain the same number of protons but different numbers of neutrons
electrically charged atom or molecule (contains unequal numbers of protons and electrons)
negatively charged atom or molecule (contains more electrons than protons)
positively charged atom or molecule (contains fewer electrons than protons)

