5.5 Isotopes of the Elements
Learning Objectives
By the end of this section, you will be able to:
- Define isotopes and identify examples of isotopes for several elements
- Write and interpret symbols that depict the atomic number, mass number of isotopes
- Write isotope names using common naming methods
What is an Isotope?
Isotopes are various forms of the same element that have the same number of protons but a different number of neutrons. As the number of neutrons of an atom changes, so does its relative isotopic mass. The relative isotopic mass (also called mass number) is the sum of the protons and neutrons present in that isotope.
Mass Number (A) = Number of Protons + Number of Neutrons
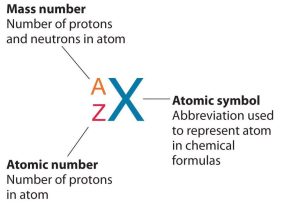
Isotope symbols for elements are used to represent specific isotopes of atoms and include mass number (A) in superscript, atomic number (Z) in subscript, followed by the element symbol (X) in normal case (Figure 5.5a).
The number of protons in the nucleus of an atom is its atomic number (Z). This is the defining trait of an element. Its atomic number (Z) determines the identity of the atom. For example, any atom that contains six protons is the element carbon and has the atomic number 6, regardless of how many neutrons or electrons it may have. If you change the atomic number to 7, you are no longer dealing with carbon atoms, but nitrogen atoms. A neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. Therefore, the atomic number also indicates the number of electrons in an atom. The total number of protons and neutrons in an atom is called its mass number (A). The number of neutrons in that atom is therefore the difference between the mass number (A) and the atomic number (Z).
In summary:
[latex]\begin{array}{r @ {{}={}} l} \text{atomic number (Z) = } & \text{number of protons} \\[1em] \text{mass number (A) = } & \text{number of protons + number of neutrons} \\[1em] \text{A - Z = } & \text{number of neutrons} \end{array}[/latex]
Examples of Isotopes and their Properties:
As mentioned above, the symbol for a specific isotope of any element is written by placing the mass number as a superscript to the left of the element symbol. The atomic number is sometimes written as a subscript preceding the symbol, but since this number defines the element’s identity (atomic number), as does its symbol, it is sometimes omitted, as shown in Figure 5.5b. The various isotopes for the element carbon and the number of each subatomic particle in that isotope are shown below:
- Carbon-12 (or 12C) has the atomic number 6 and mass number 12 (six protons and six neutrons). It contains six protons, six neutrons, and six electrons
- Carbon-13 (or 13C) has the atomic number 6 and mass number 13 (six protons and seven neutrons). It contains six protons, seven neutrons, and six electrons.
- Carbon-14 (or 14C) has the atomic number 6 and mass number 14 (six protons and eight neutrons). It contains six protons, eight neutrons, and six electrons
When reading a specific isotope symbol, it is read as “element, mass number.” For instance, in the case of magnesium, 24Mg is read as “magnesium 24,” and can be written as “magnesium-24” or “Mg-24.” All magnesium atoms have the atomic number 12, which means they have 12 protons in their nucleus. These isotopes differ only because a 24Mg atom has 12 neutrons in its nucleus, a 25Mg atom has 13 neutrons, and a 26Mg atom has 14 neutrons.
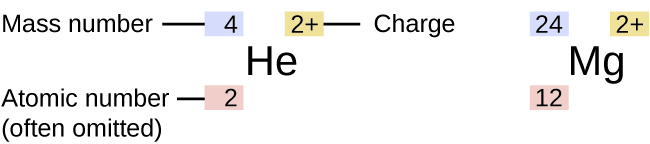
Example 5.5a
Provide the isotope short form and isotope symbol (include the mass number only, omit atomic number) for the following isotopes: Arsenic-74, Calcium-44. Refer to the periodic table if needed.
Solution
Arsenic-74: Isotope short form = As-74; isotope symbol = 74As
Calcium-44: Isotope short form = Ca-44; isotope symbol = 44Ca
Example 5.5b
Provide the correct name for the isotope having the symbol 202Hg; 56Fe. Refer to the periodic table if needed.
Solution
202Hg; Isotope name = Mercury-202
56Fe; Isotope name = Iron – 56
Example 5.5c
Write the isotope name and symbol (mass number only) for the following two elements. Refer to the periodic table if needed.
- Element 1 has 12 protons and 13 neutrons
- Element 2 has 17 protons and 20 neutrons
Solution
Element 1 has 12 protons (therefore its atomic number is 12). Using a periodic table for reference, the element with atomic number 12 is Magnesium (Mg). The mass number will be 12 p+ + 13 n0 = 25.
Isotope Name = Magnesium-25 or Mg-25
Isotope Symbol = 25Mg
Element 2 has 17 protons (therefore its atomic number is 17). Using a periodic table for reference, the element with atomic number 17 is chlorine (Cl). The mass number will be 17 p+ + 20 n0 = 37.
Isotope Name = Chlorine-37 or Cl-37
Isotope Symbol = 37Cl
Exercise 5.5a
Check Your Learning Exercise (Text Version)
Choose the option that best answers the statements for each of the provided multiple choice questions.
- Isotopes differ in the number of
- orbitals
- protons
- neutrons
- electrons
- charges
- Hydrogen has three naturally occurring isotopes. Which of the following is not an isotope of hydrogen?
- Quatrium, 4H, contains one electron, one proton, and three neutrons
- Tritium, 3H, contains one electron, one proton, and two neutrons
- Deuterium, has symbol 2H, contains one electron, one proton, and one neutron
- Protium, has symbol 1H, contains one electron, one proton, and no neutrons
- The isotope short form and isotope symbol (include the mass number only, omit atomic number) for Iodine-127 is
- I-127; 127I
- I-127; 127I
- Io-127; 127Io
- I-53; 53I
- I-74; 74I
- Which of the following is the isotope symbol (include the mass number only, omit atomic number) for the atom that has 15 protons and 16 neutrons.
- 16O
- 31Ga
- 15P
- 16P
- 31P
- The isotope name and short form for a neutral atom that has with 7 electrons and 8 neutrons is
- nitrogen – 8; N-8
- nitrogen – 14; N-14
- nitrogen – 15; N-15
- oxygen – 14; O-14
- oxygen – 15; O-15
- Tin is a silvery malleable metallic element belonging to group 14 of the periodic table. Tin has many stable naturally occurring isotopes, including tin–120, which has a natural abundance of 32.58%. Which of the following is true of the isotope tin-120?
- As a neutral isotope, it contains 50 protons, 50 electrons, and 70 neutrons
- Its mass number is 120
- Its atomic number is 50
- Its isotope symbol is 120Sn
- all of these options contain true information about tin-120
- The element X in an atom with a mass number (A) of 33 and atomic number (Z) 16 is
- Arsenic
- Indium
- Sulfur
- Chlorine
- none of these elements represent this isotope
- Which of the following is element X in an atom with a mass number of 58 and contains 30 neutrons?
- Cobalt
- Copper
- Cerium
- Nickel
- Zinc
Check Your Answer[1]
Source: “Exercise 5.5a” by Jackie MacDonald, licensed under CC BY-NC-SA 4.0.
While the atomic mass (mass number) of individual isotopes of a given element is different, their physical and chemical properties remain mostly the same. However, isotopes do differ in their stability. Carbon-12 (12C) is the most abundant of the carbon isotopes, accounting for 98.93% of carbon on Earth. Carbon-13 (13C) is stable but only accounts for about 1.07% abundance in nature. Carbon-14 (14C) isotopes are found in trace amounts in nature and are unstable or radioactive. Radioactive isotopes may decay over time by emitting neutrons, protons, and/or electrons and energy to obtain a more stable form. Further information relating to radioactivity and radioactive decay would be covered in more detail in nuclear chemistry or nuclear physics.
Information about the naturally occurring isotopes of elements with atomic numbers 1 through 10 is given in Table 5.5a. Note that in addition to standard names and symbols, the isotopes of hydrogen are often referred to using common names and accompanying symbols. Hydrogen-2, symbolized 2H, is also called deuterium and sometimes symbolized D. Hydrogen-3, symbolized 3H, is also called tritium and sometimes symbolized T.
| Isotope Name | Symbol | Atomic Number | Number of Protons | Number of Neutrons | Mass Number | Mass (amu) | % Natural Abundance |
|---|---|---|---|---|---|---|---|
| hydrogen-1 (protium) | [latex]_1^1\text{H}[/latex] | 1 | 1 | 0 | 1 | 1.0078 | 99.989 |
| hydrogen-2 (deuterium) | [latex]_1^2\text{H}[/latex] | 1 | 1 | 1 | 2 | 2.0141 | 0.0115 |
| hydrogen-3 (tritium) | [latex]_1^3\text{H}[/latex] | 1 | 1 | 2 | 3 | 3.01605 | — (trace) |
| helium-3 | [latex]_2^3\text{He}[/latex] | 2 | 2 | 1 | 3 | 3.01603 | 0.00013 |
| helium-4 | [latex]_2^4\text{He}[/latex] | 2 | 2 | 2 | 4 | 4.0026 | 100 |
| lithium-6 | [latex]_3^6\text{Li}[/latex] | 3 | 3 | 3 | 6 | 6.0151 | 7.59 |
| lithium-7 | [latex]_3^7\text{Li}[/latex] | 3 | 3 | 4 | 7 | 7.0160 | 92.41 |
| beryllium-9 | [latex]_4^9\text{Be}[/latex] | 4 | 4 | 5 | 9 | 9.0122 | 100 |
| boron-10 | [latex]_5^{10}\text{B}[/latex] | 5 | 5 | 5 | 10 | 10.0129 | 19.9 |
| boron-11 | [latex]_5^{11}\text{B}[/latex] | 5 | 5 | 6 | 11 | 11.0093 | 80.1 |
| carbon-12 | [latex]_6^{12}\text{C}[/latex] | 6 | 6 | 6 | 12 | 12.0000 | 98.89 |
| carbon-13 | [latex]_6^{13}\text{C}[/latex] | 6 | 6 | 7 | 13 | 13.0034 | 1.11 |
| carbon-14 | [latex]_6^{14}\text{C}[/latex] | 6 | 6 | 8 | 14 | 14.0032 | — (trace) |
| nitrogen-14 | [latex]_7^{14}\text{N}[/latex] | 7 | 7 | 7 | 14 | 14.0031 | 99.63 |
| nitrogen-15 | [latex]_7^{15}\text{N}[/latex] | 7 | 7 | 8 | 15 | 15.0001 | 0.37 |
| oxygen-16 | [latex]_8^{16}\text{O}[/latex] | 8 | 8 | 8 | 16 | 15.9949 | 99.757 |
| oxygen-17 | [latex]_8^{17}\text{O}[/latex] | 8 | 8 | 9 | 17 | 16.9991 | 0.038 |
| oxygen-18 | [latex]_8^{18}\text{O}[/latex] | 8 | 8 | 10 | 18 | 17.9992 | 0.205 |
| fluorine-19 | [latex]_9^{19}\text{F}[/latex] | 9 | 9 | 10 | 19 | 18.9984 | 100 |
| neon-20 | [latex]_{10}^{20}\text{Ne}[/latex] | 10 | 10 | 10 | 20 | 19.9924 | 90.48 |
| neon-21 | [latex]_{10}^{21}\text{Ne}[/latex] | 10 | 10 | 11 | 21 | 20.9938 | 0.27 |
| neon-22 | [latex]_{10}^{22}\text{Ne}[/latex] | 10 | 10 | 12 | 22 | 21.9914 | 9.25 |
Exercise 5.5b
Practice using the following PhET simulation: Isotopes and Atomic Mass
Scientists in Action: Mildred Cohn, PhD.
As you will learn, isotopes are important in nature and especially in human understanding of science and medicine. Let’s consider just one natural, stable isotope: Oxygen-18, which is noted in the Table 5.5a above and is referred to as one of the environmental isotopes. It is important in paleoclimatology, for example, because scientists can use the ratio between Oxygen-18 and Oxygen-16 in an ice core to determine the temperature of precipitation over time. Oxygen-18 (Figure 5.5c) was also critical to the discovery of metabolic pathways and the mechanisms of enzymes.
Mildred Cohn pioneered the usage of these isotopes to act as tracers, so that researchers could follow their path through reactions and gain a better understanding of what is happening. One of her first discoveries provided insight into the phosphorylation of glucose that takes place in mitochondria. And the methods of using isotopes for this research contributed to entire fields of study.
Learn more about Mildred Cohn’s work and her motivating story of overcoming gender and religious biases in the article Mildren Cohn (1913-2009) [New Tab].
WATCH Women in Chemistry: Mildred Cohn (18min 42sec).
Video Source: Science History Institute (2012, September 10). Women in Chemistry: Mildred Cohn [Video]. YouTube.
Links to Interactive Learning Tools
Explore Isotopes from the Physics Classroom.
Attribution & References
Except where otherwise noted, this page is adapted by Jackie MacDonald from “2.3 – Atomic Structure and Symbolism ” In Chemistry 2e (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson is licensed under CC BY 4.0. Access for free at Chemistry 2e (Open Stax) / Reused section on isotopes, rewrote learning objectives.
- (1) c; (2) a; (3) a; (4) e; (5) c; (6) e; (7) c; (8)d ↵
sum of the numbers of neutrons and protons in the nucleus of an atom
number of protons in the nucleus of an atom

