Chapter 17 – Review
17.1 Chemical Reaction Rates
- What is the difference between average rate, initial rate, and instantaneous rate? Check Answer: [1]
- Ozone decomposes to oxygen according to the equation [latex]2\text{ O}_3(g)\;{\longrightarrow}\;3\text{ O}_2(g)[/latex]. Write the equation that relates the rate expressions for this reaction in terms of the disappearance of O3 and the formation of oxygen.
- In the nuclear industry, chlorine trifluoride is used to prepare uranium hexafluoride, a volatile compound of uranium used in the separation of uranium isotopes. Chlorine trifluoride is prepared by the reaction [latex]\text{Cl}_2(g)\;+\;3\text{ F}_2(g)\;{\longrightarrow}\;2\text{ ClF}_3(g)[/latex]. Write the equation that relates the rate expressions for this reaction in terms of the disappearance of Cl2 and F2 and the formation of ClF3. Check Answer: [2]
- A study of the rate of dimerization of C4H6 gave the data shown in the table below:
[latex]2\text{ C}_4\text{H}_6\;{\longrightarrow}\;\text{C}_8\text{H}_{12}[/latex]Time (s) 0 1600 3200 4800 6200 [C4H6] (M) 1.00 × 10−2 5.04 × 10−3 3.37 × 10−3 2.53 × 10−3 2.08 × 10−3 - Determine the average rate of dimerization between 0 s and 1600 s, and between 1600 s and 3200 s.
- Estimate the instantaneous rate of dimerization at 3200 s from a graph of time versus [C4H6]. What are the units of this rate?
- Determine the average rate of formation of C8H12 at 1600 s and the instantaneous rate of formation at 3200 s from the rates found in parts (a) and (b).
- A study of the rate of the reaction represented as [latex]2A\;{\longrightarrow}\;B[/latex] gave the following data as shown in the table below:
Time (s) 0.0 5.0 10.0 15.0 20.0 25.0 35.0 [A] (M) 1.00 0.952 0.625 0.465 0.370 0.308 0.230 - Determine the average rate of disappearance of A between 0.0 s and 10.0 s, and between 10.0 s and 20.0 s.
- Estimate the instantaneous rate of disappearance of A at 15.0 s from a graph of time versus [A]. What are the units of this rate?
- Use the rates found in parts (a) and (b) to determine the average rate of formation of B between 0.00 s and 10.0 s, and the instantaneous rate of formation of B at 15.0 s.
Check Answer: [3]
- Consider the following reaction in aqueous solution:
[latex]5\text{ Br}^{-}(aq)\;+\;\text{BrO}_3^{\;\;-}(aq)\;+\;6\text{ H}^{+}(aq)\;{\longrightarrow}\;3\text{ Br}_2(aq)\;+\;3\text{ H}_2\text{O}(l)[/latex]If the rate of disappearance of Br–(aq) at a particular moment during the reaction is 3.5 × 10−4M s−1, what is the rate of appearance of Br2(aq) at that moment?
17.2 Chemical Equilibria
- What does it mean to describe a reaction as “reversible”? Check Answer: [4]
- When writing an equation, how is a reversible reaction distinguished from a nonreversible reaction?
- If a reaction is reversible, when can it be said to have reached equilibrium? Check Answer: [5]
- Is a system at equilibrium if the rate constants of the forward and reverse reactions are equal?
- If the concentrations of products and reactants are equal, is the system at equilibrium? Check Answer: [6]
17.3 Equilibrium Constants
- Explain why there may be an infinite number of values for the reaction quotient of a reaction at a given temperature but there can be only one value for the equilibrium constant at that temperature.
- Explain why an equilibrium between Br2(l) and Br2(g) would not be established if the container were not a closed vessel. Check Answer: [7]
- If you observe the following reaction at equilibrium, is it possible to tell whether the reaction started with pure NO2 or with pure N2O4?
[latex]2\text{ NO}_2(g)\;{\rightleftharpoons}\;\text{N}_2\text{O}_4(g)[/latex] - Among the solubility rules previously discussed is the statement: All chlorides are soluble except Hg2Cl2, AgCl, PbCl2, and CuCl.
- Write the expression for the equilibrium constant for the reaction represented by the equation [latex]\text{AgCl}(s)\;{\rightleftharpoons}\;\text{Ag}^{+}(aq)\;+\;\text{Cl}^{-}(aq)[/latex]. Is Kc > 1, < 1, or ≈ 1? Explain your answer.
- Write the expression for the equilibrium constant for the reaction represented by the equation [latex]\text{Pb}^{2+}(aq)\;+\;2\text{ Cl}^{-}(aq)\;{\rightleftharpoons}\;\text{PbCl}_2(s)[/latex]. Is Kc > 1, < 1, or ≈ 1? Explain your answer.
Check Answer: [8]
- Among the solubility rules previously discussed is the statement: Carbonates, phosphates, borates, and arsenates—except those of the ammonium ion and the alkali metals—are insoluble.
- Write the expression for the equilibrium constant for the reaction represented by the equation [latex]\text{CaCO}_3(s)\;{\rightleftharpoons}\;\text{Ca}^{2+}(aq)\;+\;\text{CO}_3^{\;\;-}(aq)[/latex]. Is Kc > 1, < 1, or ≈ 1? Explain your answer.
- Write the expression for the equilibrium constant for the reaction represented by the equation [latex]3\text{ Ba}^{2+}(aq)\;+\;2\text{ PO}_4^{\;\;3-}(aq)\;{\rightleftharpoons}\;\text{Ba}_3(\text{PO}_4)_2(s)[/latex]. Is Kc > 1, < 1, or ≈ 1? Explain your answer.
- Benzene is one of the compounds used as octane enhancers in unleaded gasoline. It is manufactured by the catalytic conversion of acetylene to benzene: [latex]3\text{ C}_2\text{H}_2(g)\;{\longrightarrow}\;\text{C}_6\text{H}_6(g)[/latex]. Which value of Kc would make this reaction most useful commercially? Kc ≈ 0.01, Kc ≈ 1, or Kc ≈ 10. Explain your answer. Check Answer: [9]
- Show that the complete chemical equation, the total ionic equation, and the net ionic equation for the reaction represented by the equation [latex]\text{KI}(aq)\;+\;\text{I}_2(aq)\;{\rightleftharpoons}\;\text{KI}_3(aq)[/latex] give the same expression for the reaction quotient. KI3 is composed of the ions K+ and I3–.
- For a titration to be effective, the reaction must be rapid and the yield of the reaction must essentially be 100%. Is Kc > 1, < 1, or ≈ 1 for a titration reaction? Check Answer: [10]
- For a precipitation reaction to be useful in a gravimetric analysis, the product of the reaction must be insoluble. Is Kc > 1, < 1, or ≈ 1 for a useful precipitation reaction?
- Write the mathematical expression for the reaction quotient, Qc, for each of the following reactions:
- [latex]\text{CH}_4(g)\;+\;\text{Cl}_2(g)\;{\rightleftharpoons}\;\text{CH}_3\text{Cl}(g)\;+\;\text{HCl}(g)[/latex]
- [latex]\text{N}_2(g)\;+\;\text{O}_2(g)\;{\rightleftharpoons}\;2\text{ NO}(g)[/latex]
- [latex]2\text{ SO}_2(g)\;+\;\text{O}_2(g)\;{\rightleftharpoons}\;2\text{ SO}_3(g)[/latex]
- [latex]\text{BaSO}_3(s)\;{\rightleftharpoons}\;\text{BaO}(s)\;+\;\text{SO}_2(g)[/latex]
- [latex]\text{P}_4(g)\;+\;5\text{ O}_2(g)\;{\rightleftharpoons}\;\text{P}_4\text{O}_{10}(s)[/latex]
- [latex]\text{Br}_2(g)\;{\rightleftharpoons}\;2\text{ Br}(g)[/latex]
- [latex]\text{CH}_4(g)\;+\;2\text{ O}_2(g)\;{\rightleftharpoons}\;\text{CO}_2(g)\;+\;2\text{ H}_2\text{O}(l)[/latex]
- [latex]\text{CuSO}_4{\cdot}5\text{H}_2\text{O}(s)\;{\rightleftharpoons}\;\text{CuSO}_4(s)\;+\;5\text{ H}_2\text{O}(g)[/latex]
Check Answer: [11]
- Write the mathematical expression for the reaction quotient, Qc, for each of the following reactions:
- [latex]\text{N}_2(g)\;+\;3\text{ H}_2(g)\;{\rightleftharpoons}\;2\text{ NH}_3(g)[/latex]
- [latex]4\text{ NH}_3(g)\;+\;5\text{ O}_2(g)\;{\rightleftharpoons}\;4\text{ NO}(g)\;+\;6\text{ H}_2\text{O}(g)[/latex]
- [latex]\text{N}_2\text{O}_4(g)\;{\rightleftharpoons}\;2\text{ NO}_2(g)[/latex]
- [latex]\text{CO}_2(g)\;+\;\text{H}_2(g)\;{\rightleftharpoons}\;\text{CO}(g)\;+\;\text{H}_2\text{O}(g)[/latex]
- [latex]\text{NH}_4\text{Cl}(s)\;{\rightleftharpoons}\;\text{NH}_3(g)\;+\;\text{HCl}(g)[/latex]
- [latex]2\text{Pb( NO}_3)_2(s)\;{\rightleftharpoons}\;2\text{ PbO}(s)\;+\;4\text{ NO}_2(g)\;+\;\text{O}_2(g)[/latex]
- [latex]2\text{ H}_2(g)\;+\;\text{O}_2(g)\;{\rightleftharpoons}\;2\text{ H}_2\text{O}(l)[/latex]
- [latex]\text{S}_8(g)\;{\rightleftharpoons}\;8\text{ S}(g)[/latex]
- The initial concentrations or pressures of reactants and products are given for each of the following systems. Calculate the reaction quotient and determine the direction in which each system will proceed to reach equilibrium.
- [latex]2\text{ NH}_3(g)\;{\rightleftharpoons}\;\text{N}_2(g)\;+\;3\text{ H}_2(g)\;\;\;\;\;\;\;K_c = 17[/latex]; [NH3] = 0.20 M, [N2] = 1.00 M, [H2] = 1.00 M
- [latex]2\text{ NH}_3(g)\;{\rightleftharpoons}\;\text{N}_2(g)\;+\;3\text{ H}_2(g)\;\;\;\;\;\;\;K_P = 6.8\;\times\;10^4[/latex]; initial pressures: NH3 = 3.0 atm, N2 = 2.0 atm, H2 = 1.0 atm
- [latex]2\text{ SO}_3(g)\;{\rightleftharpoons}\;2\text{ SO}_2(g)\;+\;\text{O}_2(g)\;\;\;\;\;\;\;K_c = 0.230[/latex]; [SO3] = 0.00 M, [SO2] = 1.00 M, [O2] = 1.00 M
- [latex]2\text{ SO}_3(g)\;{\rightleftharpoons}\;2\text{ SO}_2(g)\;+\;\text{O}_2(g)\;\;\;\;\;\;\;K_P = 16.5[/latex]; initial pressures: SO3 = 1.00 atm, SO2 = 1.00 atm, O2 = 1.00 atm
- [latex]2\text{ NO}(g)\;+\;\text{Cl}_2(g)\;{\rightleftharpoons}\;2\text{ NOCl}(g)\;\;\;\;\;\;\;K_c = 4.6\;\times\;10^4[/latex]; [NO] = 1.00 M, [Cl2] = 1.00 M, [NOCl] = 0 M
- [latex]\text{N}_2(g)\;+\;\text{O}_2(g)\;{\rightleftharpoons}\;2\text{ NO}(g)\;\;\;\;\;\;\;K_P = 0.050[/latex]; initial pressures: NO = 10.0 atm, N2 = O2 = 5 atm
Check Answer: [12]
- The initial concentrations or pressures of reactants and products are given for each of the following systems. Calculate the reaction quotient and determine the direction in which each system will proceed to reach equilibrium.
- [latex]2\text{ NH}_3(g)\;{\rightleftharpoons}\;\text{N}_2(g)\;+\;3\text{ H}_2(g)\;\;\;\;\;\;\;K_c = 17[/latex]; [NH3] = 0.50 M, [N2] = 0.15 M, [H2] = 0.12 M
- [latex]2\text{ NH}_3(g)\;{\rightleftharpoons}\;\text{N}_2(g)\;+\;3\text{ H}_2(g)\;\;\;\;\;\;\;K_P = 6.8\;\times\;10^4[/latex]; initial pressures: NH3 = 2.00 atm, N2 = 10.00 atm, H2 = 10.00 atm
- [latex]2\text{ SO}_3(g)\;{\rightleftharpoons}\;2\text{ SO}_2(g)\;+\;\text{O}_2(g)\;\;\;\;\;\;\;K_c = 0.230[/latex]; [SO3] = 2.00 M, [SO2] = 2.00 M, [O2] = 2.00 M
- [latex]2\text{ SO}_3(g)\;{\rightleftharpoons}\;2\text{ SO}_2(g)\;+\;\text{O}_2(g)\;\;\;\;\;\;\;K_P = 6.5\;\text{atm}[/latex]; initial pressures: SO2 = 1.00 atm, O2 = 1.130 atm, SO3 = 0 atm
- [latex]2\text{ NO}(g)\;+\;\text{Cl}_2(g)\;{\rightleftharpoons}\;2\text{ NOCl}(g)\;\;\;\;\;\;\;K_P = 2.5\;\times\;10^3[/latex]; initial pressures: NO = 1.00 atm, Cl2 = 1.00 atm, NOCl = 0 atm
- [latex]\text{N}_2(g)\;+\;\text{O}_2(g)\;{\rightleftharpoons}\;2\text{ NO}(g)\;\;\;\;\;\;\;K_c = 0.050[/latex]; [N2] = 0.100 M, [O2] = 0.200 M, [NO] = 1.00 M
- The following reaction has KP = 4.50 × 10−5 at 720 K.
[latex]\text{N}_2(g)\;+\;3\text{ H}_2(g)\;{\rightleftharpoons}\;2\text{ NH}_3(g)[/latex]
If a reaction vessel is filled with each gas to the partial pressures listed, in which direction will it shift to reach equilibrium? P(NH3) = 93 atm, P(N2) = 48 atm, and P(H2) = 52 Check Answer: [13] - Determine if the following system is at equilibrium. If not, in which direction will the system need to shift to reach equilibrium?
[latex]\text{SO}_2\text{Cl}_2(g)\;{\rightleftharpoons}\;\text{SO}_2(g)\;+\;\text{Cl}_2(g)[/latex]
[SO2Cl2] = 0.12 M, [Cl2] = 0.16 M and [SO2] = 0.050 M. Kc for the reaction is 0.078. - Convert the values of Kc to values of KP or the values of KP to values of Kc.
- [latex]\text{N}_2(g)\;+\;3\text{ H}_2(g)\;{\rightleftharpoons}\;2\text{ NH}_3(g)\;\;\;\;\;\;\;K_c = 0.50\;\text{at}\;400\;^{\circ}\text{C}[/latex]
- [latex]\text{H}_2\;+\;\text{I}_2\;{\rightleftharpoons}\;2\text{ HI}\;\;\;\;\;\;\;K_c = 50.2\;\text{at}\;448\;^{\circ}\text{C}[/latex]
- [latex]\text{Na}_2\text{SO}_4{\cdot}10\text{H}_2\text{O}(s)\;{\rightleftharpoons}\;\text{Na}_2\text{SO}_4(s)\;+\;10\text{ H}_2\text{O}(g)\;\;\;\;\;\;\;K_P = 4.08\;\times\;10^{-25}\;\text{at}\;25\;^{\circ}\text{C}[/latex]
- [latex]\text{H}_2\text{O}(l)\;{\rightleftharpoons}\;\text{H}_2\text{O}(g)\;\;\;\;\;\;\;K_P = 0.122\;\text{at}\;50\;^{\circ}\text{C}[/latex]
Check Answer: [14]
- Convert the values of Kc to values of KP or the values of KP to values of Kc.
- [latex]\text{Cl}_2(g)\;+\;\text{Br}_2(g)\;{\rightleftharpoons}\;2\text{ BrCl}(g)\;\;\;\;\;\;\;K_c = 4.7\;\times\;10^{-2}\;\text{at}\;25\;^{\circ}\text{C}[/latex]
- [latex]2\text{ SO}_2(g)\;+\;\text{O}_2(g)\;{\rightleftharpoons}\;2\text{ SO}_3(g)\;\;\;\;\;\;\;K_P = 48.2\;\text{at}\;500\;^{\circ}\text{C}[/latex]
- [latex]\text{CaCl}_2{\cdot}6\text{H}_2\text{O}(s)\;{\rightleftharpoons}\;\text{CaCl}_2(s)\;+\;6\text{ H}_2\text{O}(g)\;\;\;\;\;\;\;K_P = 5.09\;\times\;10^{-44}\;\text{at}\;25\;^{\circ}\text{C}[/latex]
- [latex]\text{H}_2\text{O}(l)\;{\rightleftharpoons}\;\text{H}_2\text{O}(g)\;\;\;\;\;\;\;K_P = 0.196\;\text{at}\;60\;^{\circ}\text{C}[/latex]
- What is the value of the equilibrium constant expression for the change [latex]\text{H}_2\text{O}(l)\;{\rightleftharpoons}\;\text{H}_2\text{O}(g)[/latex] at 30 °C? Check Answer: [15]
- Write the expression of the reaction quotient for the ionization of HOCN in water.
- Write the reaction quotient expression for the ionization of NH3 in water.Check Answer: [16]
- What is the approximate value of the equilibrium constant KP for the change [latex]\text{C}_2\text{H}_5\text{OC}_2\text{H}_5(l)\;{\rightleftharpoons}\;\text{C}_2\text{H}_5\text{OC}_2\text{H}_5(g)[/latex] at 25 °C. (Vapour pressure was described in the previous chapter on liquids and solids; refer back to this chapter to find the relevant information needed to solve this problem.)
17.4 Shifting Equilibria: Le Châtelier’s Principle
- The following equation represents a reversible decomposition:
[latex]\text{CaCO}_3(s)\;{\rightleftharpoons}\;\text{CaO}(s)\;+\;\text{CO}_2(g)[/latex]
Under what conditions will decomposition in a closed container proceed to completion so that no CaCO3 remains? Check Answer: [17] - Explain how to recognize the conditions under which changes in pressure would affect systems at equilibrium.
- What property of a reaction can we use to predict the effect of a change in temperature on the value of an equilibrium constant? Check Answer: [18]
- What would happen to the colour of the solution in part (b) of Figure 17.4a if a small amount of NaOH were added and Fe(OH)3 precipitated? Explain your answer.
- The following reaction occurs when a burner on a gas stove is lit:
[latex]\text{CH}_4(g)\;+\;2\text{ O}_2(g)\;{\rightleftharpoons}\;\text{CO}_2(g)\;+\;2\text{ H}_2\text{O}(g)[/latex]
Is an equilibrium among CH4, O2, CO2, and H2O established under these conditions? Explain your answer. Check Answer: [19] - A necessary step in the manufacture of sulfuric acid is the formation of sulfur trioxide, SO3, from sulfur dioxide, SO2, and oxygen, O2, shown here. At high temperatures, the rate of formation of SO3 is higher, but the equilibrium amount (concentration or partial pressure) of SO3 is lower than it would be at lower temperatures.
[latex]2\text{ SO}_2(g)\;+\;\text{O}_2(g)\;{\longrightarrow}\;2\text{ SO}_3(g)[/latex]- Does the equilibrium constant for the reaction increase, decrease, or remain about the same as the temperature increases?
- Is the reaction endothermic or exothermic?
- Suggest four ways in which the concentration of PH3 could be increased in an equilibrium described by the following equation:
[latex]\text{P}_4(g)\;+\;6\text{ H}_2(g)\;{\rightleftharpoons}\;4\text{ PH}_3(g)\;\;\;\;\;\;\;{\Delta}H = 110.5\;\text{kJ}[/latex]
Check Answer: [20] - Water gas is a 1:1 mixture of carbon monoxide and hydrogen gas and is called water gas because it is formed from steam and hot carbon in the following reaction: [latex]\text{H}_2\text{ O}(g)\;+\;\text{C}(s)\;{\rightleftharpoons}\;\text{H}_2(g)\;+\;\text{CO}(g)[/latex]. Methanol, a liquid fuel that could possibly replace gasoline, can be prepared from water gas and hydrogen at high temperature and pressure in the presence of a suitable catalyst.
- Write the expression for the equilibrium constant (Kc) for the reversible reaction
[latex]2\text{H}_2(g)\;+\;\text{CO}(g)\;{\rightleftharpoons}\;\text{CH}_3\text{OH}(g)\;\;\;\;\;\;\;{\Delta}H = -90.2\;\text{kJ}[/latex] - What will happen to the concentrations of H2, CO, and CH3OH at equilibrium if more H2 is added?
- What will happen to the concentrations of H2, CO, and CH3OH at equilibrium if CO is removed?
- What will happen to the concentrations of H2, CO, and CH3OH at equilibrium if CH3OH is added?
- What will happen to the concentrations of H2, CO, and CH3OH at equilibrium if the temperature of the system is increased?
- What will happen to the concentrations of H2, CO, and CH3OH at equilibrium if more catalyst is added?
- Write the expression for the equilibrium constant (Kc) for the reversible reaction
- Nitrogen and oxygen react at high temperatures.
- Write the expression for the equilibrium constant (Kc) for the reversible reaction
[latex]\text{N}_2(g)\;+\;\text{O}_2(g)\;{\rightleftharpoons}\;2\text{ NO}(g)\;\;\;\;\;\;\;{\Delta}H = 181\;\text{kJ}[/latex] - What will happen to the concentrations of N2, O2, and NO at equilibrium if more O2 is added?
- What will happen to the concentrations of N2, O2, and NO at equilibrium if N2 is removed?
- What will happen to the concentrations of N2, O2, and NO at equilibrium if NO is added?
- What will happen to the concentrations of N2, O2, and NO at equilibrium if the pressure on the system is increased by reducing the volume of the reaction vessel?
- What will happen to the concentrations of N2, O2, and NO at equilibrium if the temperature of the system is increased?
- What will happen to the concentrations of N2, O2, and NO at equilibrium if a catalyst is added?
Check Answer:
- Write the expression for the equilibrium constant (Kc) for the reversible reaction
- Water gas, a mixture of H2 and CO, is an important industrial fuel produced by the reaction of steam with red hot coke, essentially pure carbon.
- Write the expression for the equilibrium constant for the reversible reaction
[latex]\text{C}(s)\;+\;\text{H}_2\text{O}(g)\;{\rightleftharpoons}\;\text{CO}(g)\;+\;\text{H}_2(g)\;\;\;\;\;\;\;{\Delta}H = 131.30\;\text{kJ}[/latex] - What will happen to the concentration of each reactant and product at equilibrium if more C is added?
- What will happen to the concentration of each reactant and product at equilibrium if H2O is removed?
- What will happen to the concentration of each reactant and product at equilibrium if CO is added?
- What will happen to the concentration of each reactant and product at equilibrium if the temperature of the system is increased?
- Write the expression for the equilibrium constant for the reversible reaction
- Pure iron metal can be produced by the reduction of iron(III) oxide with hydrogen gas.
- Write the expression for the equilibrium constant (Kc) for the reversible reaction
[latex]\text{Fe}_2\text{O}_3(s)\;+\;3\text{ H}_2(g)\;{\rightleftharpoons}\;2\text{ Fe}(s)\;+\;3\text{ H}_2\text{O}(g)\;\;\;\;\;\;\;{\Delta}H = 98.7\;\text{kJ}[/latex] - What will happen to the concentration of each reactant and product at equilibrium if more Fe is added?
- What will happen to the concentration of each reactant and product at equilibrium if H2O is removed?
- What will happen to the concentration of each reactant and product at equilibrium if H2 is added?
- What will happen to the concentration of each reactant and product at equilibrium if the pressure on the system is increased by reducing the volume of the reaction vessel?
- What will happen to the concentration of each reactant and product at equilibrium if the temperature of the system is increased?
Check Answer: [21]
- Write the expression for the equilibrium constant (Kc) for the reversible reaction
- Ammonia is a weak base that reacts with water according to this equation:
[latex]\text{NH}_3(aq)\;+\;\text{H}_2\text{O}(l)\;{\rightleftharpoons}\;\text{NH}_4^{\;\;+}(aq)\;+\;\text{OH}^{-}(aq)[/latex]
Will any of the following increase the percent of ammonia that is converted to the ammonium ion in water?- Addition of NaOH
- Addition of HCl
- Addition of NH4Cl
- Acetic acid is a weak acid that reacts with water according to this equation:
[latex]\text{CH}_3\text{CO}_2\text{H}(aq)\;+\;\text{H}_2\text{O}(aq)\;{\rightleftharpoons}\;\text{H}_3\text{O}^{+}(aq)\;+\;\text{CH}_3\text{CO}_2^{\;\;-}(aq)[/latex]
Will any of the following increase the percent of acetic acid that reacts and produces CH3CO2– ion?- Addition of HCl
- Addition of NaOH
- Addition of NaCH3CO2
Check Answer: [22]
- Suggest two ways in which the equilibrium concentration of Ag+ can be reduced in a solution of Na+, Cl−, Ag+, and NO3– , in contact with solid AgCl.
[latex]\text{Na}^{+}(aq)\;+\;\text{Cl}^{-}(aq)\;+\;\text{Ag}^{+}(aq)\;+\;\text{NO}_3^{\;\;-}(aq)\;{\rightleftharpoons}\;\text{AgCl}(s)\;+\;\text{Na}^{+}(aq)\;+\;\text{NO}_3^{\;\;-}(aq)[/latex]
[latex]{\Delta}H = -65.9\;\text{kJ}[/latex] - How can the pressure of water vapor be increased in the following equilibrium?
[latex]\text{H}_2\text{O}(l)\;{\rightleftharpoons}\;\text{H}_2\text{O}(g)\;\;\;\;\;\;\;{\Delta}H = 41\;\text{kJ}[/latex]
Check Answer: [23] - Additional solid silver sulfate, a slightly soluble solid, is added to a solution of silver ion and sulfate ion at equilibrium with solid silver sulfate.
[latex]2\text{ Ag}^{+}(aq)\;+\;\text{SO}_4^{\;\;2-}(aq)\;{\rightleftharpoons}\;\text{Ag}_2\text{SO}_4(s)[/latex]
Which of the following will occur?- Ag+ or SO42- concentrations will not change.
- The added silver sulfate will dissolve.
- Additional silver sulfate will form and precipitate from solution as Ag+ ions and SO42- ions combine.
- The Ag+ ion concentration will increase and the SO42- ion concentration will decrease.
- The amino acid alanine has two isomers, α-alanine and β-alanine. When equal masses of these two compounds are dissolved in equal amounts of a solvent, the solution of α-alanine freezes at the lowest temperature. Which form, α-alanine or β-alanine, has the larger equilibrium constant for ionization [latex](\text{HX}\;{\rightleftharpoons}\;\text{H}^{+}\;+\;\text{X}^{-})[/latex]?
Check Answer: [24]
17.5 Equilibrium Calculations
- A reaction is represented by this equation: [latex]\text{A}(aq)\;+\;2\text{ B}(aq)\;{\rightleftharpoons}\;2\text{ C}(aq)\;\;\;\;\;\;\;K_c = 1\;\times\;10^3[/latex]
- Write the mathematical expression for the equilibrium constant.
- Using concentrations ≤1 M, make up two sets of concentrations that describe a mixture of A, B, and C at equilibrium.
Check Answer: [25]
- A reaction is represented by this equation: [latex]2\text{ W}(aq)\;{\rightleftharpoons}\;\text{X}(aq)\;+\;2\text{ Y}(aq)\;\;\;\;\;\;\;K_c = 5\;\times\;10^{-4}[/latex]
- Write the mathematical expression for the equilibrium constant.
- Using concentrations of ≤1 M, make up two sets of concentrations that describe a mixture of W, X, and Y at equilibrium.
- What is the value of the equilibrium constant at 500 °C for the formation of NH3 according to the following equation?
[latex]\text{N}_2(g)\;+\;3\text{ H}_2(g)\;{\rightleftharpoons}\;2\text{ NH}_3(g)[/latex]
An equilibrium mixture of NH3(g), H2(g), and N2(g) at 500 °C was found to contain 1.35 M H2, 1.15 M N2, and 4.12 × 10−1M NH3.
Check Answer: [26] - Hydrogen is prepared commercially by the reaction of methane and water vapour at elevated temperatures.
[latex]\text{CH}_4(g)\;+\;\text{H}_2\text{O}(g)\;{\rightleftharpoons}\;3\text{ H}_2(g)\;+\;\text{CO}(g)[/latex]
What is the equilibrium constant for the reaction if a mixture at equilibrium contains gases with the following concentrations: CH4, 0.126 M; H2O, 0.242 M; CO, 0.126 M; H2 1.15 M, at a temperature of 760 °C? - A 0.72-mol sample of PCl5 is put into a 1.00-L vessel and heated. At equilibrium, the vessel contains 0.40 mol of PCl3(g) and 0.40 mol of Cl2(g). Calculate the value of the equilibrium constant for the decomposition of PCl5 to PCl3 and Cl2 at this temperature. Check Answer: [27]
- At 1 atm and 25 °C, NO2 with an initial concentration of 1.00 M is 3.3 × 10−3% decomposed into NO and O2. Calculate the value of the equilibrium constant for the reaction.
[latex]2\text{ NO}_2(g)\;{\rightleftharpoons}\;2\text{ NO}(g)\;+\;\text{O}_2(g)[/latex] - Calculate the value of the equilibrium constant KP for the reaction [latex]2\text{ NO}(g)\;+\;\text{Cl}_2(g)\;{\rightleftharpoons}\;2\text{ NOCl}(g)[/latex] from these equilibrium pressures: NO, 0.050 atm; Cl2, 0.30 atm; NOCl, 1.2 atm. Check Answer: [28]
- When heated, iodine vapor dissociates according to this equation:
[latex]\text{I}_2(g)\;{\rightleftharpoons}\;2\text{ I}(g)[/latex]
At 1274 K, a sample exhibits a partial pressure of I2 of 0.1122 atm and a partial pressure due to I atoms of 0.1378 atm. Determine the value of the equilibrium constant, KP, for the decomposition at 1274 K. - A sample of ammonium chloride was heated in a closed container.
[latex]\text{NH}_4\text{Cl}(s)\;{\rightleftharpoons}\;\text{NH}_3(g)\;+\;\text{HCl}(g)[/latex]
At equilibrium, the pressure of NH3(g) was found to be 1.75 atm. What is the value of the equilibrium constant KP for the decomposition at this temperature? Check Answer: [29] - At a temperature of 60 °C, the vapour pressure of water is 0.196 atm. What is the value of the equilibrium constant KP for the transformation at 60 °C?
[latex]\text{H}_2\text{O}(l)\;{\rightleftharpoons}\;\text{H}_2\text{O}(g)[/latex] - Complete the changes in concentrations (or pressure, if requested) for each of the following reactions in the table below.
a. 2 SO3 (g) [latex]{\rightleftharpoons}[/latex] 2 SO2 (g) + O2 (g) ______ ______ +x ______ ______ 0.125 M b. 4 NH3 (g) + 3 O2 (g) [latex]{\rightleftharpoons}[/latex] 2 N2 (g) + 6 H2O (g) ______ 3x ______ ______ ______ 0.24 M ______ ______ c. 2 CH4 (g) [latex]{\rightleftharpoons}[/latex] C2H2 (g) + 3 H2 (g) ______ x ______ ______ 25 torr ______ d. CH4 (g) + H2O (g) [latex]{\rightleftharpoons}[/latex] CO (g) + 3 H2 (g) ______ x ______ ______ ______ 5 atm ______ ______ e. NH4Cl (s) [latex]{\rightleftharpoons}[/latex] NH3 (g) + HCl (g) x ______ 1.03 x 10-4 M ______ f. Ni (s) + 4 CO (g) [latex]{\rightleftharpoons}[/latex] Ni(CO)4 (g) 4x ______ 0.40 atm ______ Check Answer: [30]
- Complete the changes in concentrations (or pressure, if requested) for each of the following reactions in the table below.
a. 2 H2 (g) + O2 (g) [latex]{\rightleftharpoons}[/latex] 2 H2O (g) ______ ______ +2x ______ ______ 1.50 M b. CS2 (g) + 4 H2 (g) [latex]{\rightleftharpoons}[/latex] CH4 (g) + 2 H2S (g) x ______ ______ ______ 0.020 M ______ ______ ______ c. H2 (g) + Cl2 (g) [latex]{\rightleftharpoons}[/latex] 2 HCl (g) x ______ ______ 1.50 atm ______ ______ d. 2 NH3 (g) + 2 O2 (g) [latex]{\rightleftharpoons}[/latex] N2O (g) + 3 H2O (g) ______ ______ ______ x ______ ______ ______ 60.6 torr e. NH4HS (s) [latex]{\rightleftharpoons}[/latex] NH3 (g) + H2S (g) x ______ 9.8 x 10-6 M ______ f. Fe (s) + 5 CO (g) [latex]{\rightleftharpoons}[/latex] Fe(CO)4 (g) ______ x ______ 0.012 atm - Why are there no changes specified for Ni in Exercise 11, part (f) above? What property of Ni does change? Check Answer: [31]
- Why are there no changes specified for NH4HS in Exercise 12, part (e) above? What property of NH4HS does change?
- Analysis of the gases in a sealed reaction vessel containing NH3, N2, and H2 at equilibrium at 400 °C established the concentration of N2 to be 1.2 M and the concentration of H2 to be 0.24 M.
[latex]\text{N}_2(g)\;+\;3\text{ H}_2(g)\;{\rightleftharpoons}\;2\text{ NH}_3(g)\;\;\;\;\;\;\;K_c = 0.50\;\text{at}\;400\;^{\circ}\text{C}[/latex]
Calculate the equilibrium molar concentration of NH3. Check Answer: [32] - Calculate the number of moles of HI that are at equilibrium with 1.25 mol of H2 and 1.25 mol of I2 in a 5.00−L flask at 448 °C.
[latex]\text{H}_2\;+\;\text{I}_2\;{\rightleftharpoons}\;2\text{ HI}\;\;\;\;\;\;\;K_c = 50.2\;\text{at}\;448\;^{\circ}\text{C}[/latex] - What is the pressure of BrCl in an equilibrium mixture of Cl2, Br2, and BrCl if the pressure of Cl2 in the mixture is 0.115 atm and the pressure of Br2 in the mixture is 0.450 atm?
[latex]\text{Cl}_2(g)\;+\;\text{Br}_2(g)\;{\rightleftharpoons}\;2\text{ BrCl}(g)\;\;\;\;\;\;\;K_P = 4.7\;\times\;10^{-2}[/latex]
Check Answer: [33] - What is the pressure of CO2 in a mixture at equilibrium that contains 0.50 atm H2, 2.0 atm of H2O, and 1.0 atm of CO at 990 °C?
[latex]\text{H}_2(g)\;+\;\text{CO}_2(g)\;{\rightleftharpoons}\;\text{H}_2\text{O}(g)\;+\;\text{CO}(g)\;\;\;\;\;\;\;K_P = 1.6\;\text{at}\;990\;^{\circ}\text{C}[/latex] - Cobalt metal can be prepared by reducing cobalt(II) oxide with carbon monoxide.
[latex]\text{CoO}(s)\;+\;\text{CO}(g)\;{\rightleftharpoons}\;\text{Co}(s)\;+\;\text{CO}_2(g)\;\;\;\;\;\;\;K_c = 4.90\;\times\;10^2\;\text{at}\;550\;^{\circ}\text{C}[/latex]
What concentration of CO remains in an equilibrium mixture with [CO2] = 0.100 M? Check Answer: [34] - Carbon reacts with water vapour at elevated temperatures.
[latex]\text{C}(s)\;+\;\text{H}_2\text{O}(g)\;{\rightleftharpoons}\;\text{CO}(g)\;+\;\text{H}_2(g)\;\;\;\;\;\;\;K_c = 0.2\;\text{at}\;1000\;^{\circ}\text{C}[/latex]
What is the concentration of CO in an equilibrium mixture with [H2O] = 0.500 M at 1000 °C? - Sodium sulfate 10−hydrate, Na2SO4·10H2O, dehydrates according to the equation
[latex]\text{Na}_2\text{SO}_4{\cdot}10\text{H}_2\text{O}(s)\;{\rightleftharpoons}\;\text{Na}_2\text{SO}_4(s)\;+\;10\text{ H}_2\text{O}(g)\;\;\;\;\;\;\;K_P = 4.08\;\times\;10^{-25}\;\text{at}\;25\;^{\circ}\text{C}[/latex]
What is the pressure of water vapour at equilibrium with a mixture of Na2SO4·10H2O and NaSO4? Check Answer: [35] - Calcium chloride 6−hydrate, CaCl2·6H2O, dehydrates according to the equation
[latex]\text{CaCl}_2{\cdot}6\text{H}_2\text{O}(s)\;{\rightleftharpoons}\;\text{CaCl}_2(s)\;+\;6\text{ H}_2\text{O}(g)\;\;\;\;\;\;\;K_P = 5.09\;\times\;10^{-44}\;\text{at}\;25\;^{\circ}\text{C}[/latex]
What is the pressure of water vapour at equilibrium with a mixture of CaCl2·6H2O and CaCl2? - A student solved the following problem and found the equilibrium concentrations to be [SO2] = 0.590 M, [O2] = 0.0450 M, and [SO3] = 0.260 M. How could this student check the work without reworking the problem? The problem was: For the following reaction at 600 °C:
[latex]2\text{ SO}_2(g)\;+\;\text{O}_2(g)\;{\rightleftharpoons}\;2\text{ SO}_3(g)\;\;\;\;\;\;\;K_c = 4.32[/latex]
What are the equilibrium concentrations of all species in a mixture that was prepared with [SO3] = 0.500 M, [SO2] = 0 M, and [O2] = 0.350 M? Check Answer: [36] - A student solved the following problem and found [N2O4] = 0.16 M at equilibrium. How could this student recognize that the answer was wrong without reworking the problem? The problem was: What is the equilibrium concentration of N2O4 in a mixture formed from a sample of NO2 with a concentration of 0.10 M?
[latex]2\text{ NO}_2(g)\;{\rightleftharpoons}\;\text{N}_2\text{O}_4(g)\;\;\;\;\;\;\;K_c = 160[/latex] - Assume that the change in concentration of N2O4 is small enough to be neglected in the following problem.
- Calculate the equilibrium concentration of both species in 1.00 L of a solution prepared from 0.129 mol of N2O4 with chloroform as the solvent.
[latex]\text{N}_2\text{O}_4(g)\;{\leftrightharpoons}\;2\text{ NO}_2(g)\;\;\;\;\;\;\;K_c = 1.07\;\times\;10^{-5}[/latex] in chloroform - Show that the change is small enough to be neglected.
Check Answer: [37]
- Calculate the equilibrium concentration of both species in 1.00 L of a solution prepared from 0.129 mol of N2O4 with chloroform as the solvent.
- Assume that the change in concentration of COCl2 is small enough to be neglected in the following problem.
- Calculate the equilibrium concentration of all species in an equilibrium mixture that results from the decomposition of COCl2 with an initial concentration of 0.3166 M.
[latex]\text{COCl}_2(g)\;{\rightleftharpoons}\;\text{CO}(g)\;+\;\text{Cl}_2(g)\;\;\;\;\;\;\;K_c = 2.2\;\times\;10^{-10}[/latex] - Show that the change is small enough to be neglected.
- Calculate the equilibrium concentration of all species in an equilibrium mixture that results from the decomposition of COCl2 with an initial concentration of 0.3166 M.
- Assume that the change in pressure of H2S is small enough to be neglected in the following problem.
- Calculate the equilibrium pressures of all species in an equilibrium mixture that results from the decomposition of H2S with an initial pressure of 0.824 atm.
[latex]2\text{ H}_2\text{S}(g)\;{\rightleftharpoons}\;2\text{ H}_2(g)\;+\;\text{S}_2(g)\;\;\;\;\;\;\;K_P = 2.2\;\times\;10^{-6}[/latex] - Show that the change is small enough to be neglected. Check Answer: [38]
- Calculate the equilibrium pressures of all species in an equilibrium mixture that results from the decomposition of H2S with an initial pressure of 0.824 atm.
- What are all concentrations after a mixture that contains [H2O] = 1.00 M and [Cl2O] = 1.00 M comes to equilibrium at 25 °C?
[latex]\text{H}_2\text{O}(g)\;+\;\text{Cl}_2\text{O}(g)\;{\rightleftharpoons}\;2\text{ HOCl}(g)\;\;\;\;\;\;\;K_c = 0.0900[/latex] - What are the concentrations of PCl5, PCl3, and Cl2 in an equilibrium mixture produced by the decomposition of a sample of pure PCl5 with [PCl5] = 2.00 M?
[latex]\text{PCl}_5(g)\;{\rightleftharpoons}\;\text{PCl}_3(g)\;+\;\text{Cl}_2(g)\;\;\;\;\;\;\;K_c = 0.0211[/latex]
Check Answer: [39] - Calculate the pressures of all species at equilibrium in a mixture of NOCl, NO, and Cl2 produced when a sample of NOCl with a pressure of 10.0 atm comes to equilibrium according to this reaction:
[latex]2\text{ NOCl}(g)\;{\rightleftharpoons}\;2\text{ NO}(g)\;+\;\text{Cl}_2(g)\;\;\;\;\;\;\;K_P = 4.0\;\times\;10^{-4}[/latex] - Calculate the equilibrium concentrations of NO, O2, and NO2 in a mixture at 250 °C that results from the reaction of 0.20 M NO and 0.10 M O2. (Hint: K is large; assume the reaction goes to completion then comes back to equilibrium.) Check Answer: [40]
[latex]2\text{ NO}(g)\;+\;\text{O}_2(g)\;{\rightleftharpoons}\;2\text{ NO}_2(g)\;\;\;\;\;\;\;K_c = 2.3\;\times\;10^5\;\text{at}\;250\;^{\circ}\text{C}[/latex] - Calculate the equilibrium concentrations that result when 0.25 M O2 and 1.0 M HCl react and come to equilibrium.
[latex]4\text{ HCl}(g)\;+\;\text{O}_2(g)\;{\rightleftharpoons}\;2\text{ Cl}_2(g)\;+\;2\text{ H}_2\text{O}(g)\;\;\;\;\;\;\;K_c = 3.1\;\times\;10^{13}[/latex] - One of the important reactions in the formation of smog is represented by the equation
[latex]\text{O}_3(g)\;+\;\text{NO}(g)\;{\rightleftharpoons}\;\text{NO}_2(g)\;+\;\text{O}_2(g)\;\;\;\;\;\;\;K_P = 6.0\;\times\;10^{34}[/latex]
What is the pressure of O3 remaining after a mixture of O3 with a pressure of 1.2 × 10−8 atm and NO with a pressure of 1.2 × 10−8 atm comes to equilibrium? (Hint: KP is large; assume the reaction goes to completion then comes back to equilibrium.) Check Answer: [41] - Calculate the pressures of NO, Cl2, and NOCl in an equilibrium mixture produced by the reaction of a starting mixture with 4.0 atm NO and 2.0 atm Cl2. (Hint: KP is small; assume the reverse reaction goes to completion then comes back to equilibrium.)
[latex]2\text{ NO}(g)\;+\;\text{Cl}_2(g)\;{\rightleftharpoons}\;2\text{ NOCl}(g)\;\;\;\;\;\;\;K_P = 2.5\;\times\;10^3[/latex] - Calculate the number of grams of HI that are at equilibrium with 1.25 mol of H2 and 63.5 g of iodine at 448 °C.
[latex]\text{H}_2\;+\;\text{I}_2\;{\rightleftharpoons}\;2\text{ HI}\;\;\;\;\;\;\;K_c = 50.2\;\text{at}\;448\;^{\circ}\text{C}[/latex]
Check Answer: [42] - Butane exists as two isomers, n−butane and isobutane shown in the figure below with KP = 2.5 at 25 °C.
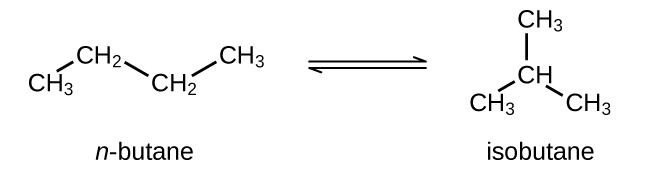
What is the pressure of isobutane in a container of the two isomers at equilibrium with a total pressure of 1.22 atm?
- What is the minimum mass of CaCO3 required to establish equilibrium at a certain temperature in a 6.50-L container if the equilibrium constant (Kc) is 0.050 for the decomposition reaction of CaCO3 at that temperature?
[latex]\text{CaCO}_3(s)\;{\rightleftharpoons}\;\text{CaO}(s)\;+\;\text{CO}_2(g)[/latex]
Check Answer: [43] - The equilibrium constant (Kc) for this reaction is 1.60 at 990 °C:
[latex]\text{H}_2(g)\;+\;\text{CO}_2(g)\;{\rightleftharpoons}\;\text{H}_2\text{O}(g)\;+\;\text{CO}(g)[/latex]
Calculate the number of moles of each component in the final equilibrium mixture obtained from adding 1.00 mol of H2, 2.00 mol of CO2, 0.750 mol of H2O, and 1.00 mol of CO to a 5.00-L container at 990 °C. - At 25 °C and at 1 atm, the partial pressures in an equilibrium mixture of N2O4 and NO2 are [latex]\text{P}_{\text{N}_2\text{O}_4} = 0.70\;\text{atm}[/latex] and [latex]\text{P}_{\text{NO}_2} = 0.30\;\text{atm}[/latex].
- Predict how the pressures of NO2 and N2O4 will change if the total pressure increases to 9.0 atm. Will they increase, decrease, or remain the same?
- Calculate the partial pressures of NO2 and N2O4 when they are at equilibrium at 9.0 atm and 25 °C. Check Answer: [44]
- In a 3.0-L vessel, the following equilibrium partial pressures are measured: N2, 190 torr; H2, 317 torr; NH3, 1.00 × 103 torr.
[latex]\text{N}_2(g)\;+\;3\text{ H}_2(g)\;{\rightleftharpoons}\;2\text{NH}_3(g)[/latex]- How will the partial pressures of H2, N2, and NH3 change if H2 is removed from the system? Will they increase, decrease, or remain the same?
- Hydrogen is removed from the vessel until the partial pressure of nitrogen, at equilibrium, is 250 torr. Calculate the partial pressures of the other substances under the new conditions.
- The equilibrium constant (Kc) for this reaction is 5.0 at a given temperature.
[latex]\text{CO}(g)\;+\;\text{H}_2\text{O}(g)\;{\rightleftharpoons}\;\text{CO}_2(g)\;+\;\text{H}_2(g)[/latex]- On analysis, an equilibrium mixture of the substances present at the given temperature was found to contain 0.20 mol of CO, 0.30 mol of water vapour, and 0.90 mol of H2 in a litre. How many moles of CO2 were there in the equilibrium mixture?
- Maintaining the same temperature, additional H2 was added to the system, and some water vapour was removed by drying. A new equilibrium mixture was thereby established containing 0.40 mol of CO, 0.30 mol of water vapour, and 1.2 mol of H2 in a litre. How many moles of CO2 were in the new equilibrium mixture? Compare this with the quantity in part (a), and discuss whether the second value is reasonable. Explain how it is possible for the water vapour concentration to be the same in the two equilibrium solutions even though some vapour was removed before the second equilibrium was established. Check Answer: [45]
- Antimony pentachloride decomposes according to this equation:
[latex]\text{SbCl}_5(g)\;{\rightleftharpoons}\;\text{SbCl}_3(g)\;+\;\text{Cl}_2(g)[/latex]
An equilibrium mixture in a 5.00-L flask at 448 °C contains 3.85 g of SbCl5, 9.14 g of SbCl3, and 2.84 g of Cl2. How many grams of each will be found if the mixture is transferred into a 2.00-L flask at the same temperature? - Consider the reaction between H2 and O2 at 1000 K
[latex]2\text{ H}_2(g)\;+\;\text{O}_2(g)\;{\rightleftharpoons}\;2\text{ H}_2\text{O}(g)\;\;\;\;\;\;\;K_P = \frac{(P_{\text{H}_2\text{O}})^2}{(P_{\text{O}_2})(P_{\text{H}_2})^3} = 1.33\;\times\;10^{20}[/latex]
If 0.500 atm of H2 and 0.500 atm of O2 are allowed to come to equilibrium at this temperature, what are the partial pressures of the components? Check Answer: [46] - An equilibrium is established according to the following equation
[latex]\text{Hg}_2^{\;\;2+}(aq)\;+\;\text{NO}_3^{\;\;-}(aq)\;+\;3\text{ H}^{+}(aq)\;{\rightleftharpoons}\;2\text{ Hg}^{2+}(aq)\;+\;\text{HNO}_2(aq)\;+\;\text{H}_2\text{O}(l)\;\;\;\;\;\;\;K_c = 4.6[/latex]
What will happen in a solution that is 0.20 M each in Hg22+, NO3–, H+, Hg2+, and HNO2?- Hg22+ will be oxidized and NO3– reduced.
- Hg22+ will be reduced and NO3– oxidized.
- Hg2+ will be oxidized and HNO2 reduced.
- Hg2+ will be reduced and HNO2 oxidized.
- There will be no change because all reactants and products have an activity of 1.
- Consider the equilibrium
[latex]4\text{ NO}_2(g)\;+\;6\text{ H}_2\text{O}(g)\;{\rightleftharpoons}\;4\text{ NH}_3(g)\;+\;7\text{ O}_2(g)[/latex]- What is the expression for the equilibrium constant (Kc) of the reaction?
- How must the concentration of NH3 change to reach equilibrium if the reaction quotient is less than the equilibrium constant?
- If the reaction were at equilibrium, how would a decrease in pressure (from an increase in the volume of the reaction vessel) affect the pressure of NO2?
- If the change in the pressure of NO2 is 28 torr as a mixture of the four gases reaches equilibrium, how much will the pressure of O2 change? Check Answer: [47]
- The binding of oxygen by hemoglobin (Hb), giving oxyhemoglobin (HbO2), is partially regulated by the concentration of H3O+ and dissolved CO2 in the blood. Although the equilibrium is complicated, it can be summarized as
[latex]\text{HbO}_2(aq)\;+\;\text{H}_3\text{O}^{+}(aq)\;+\;\text{CO}_2(g)\;{\rightleftharpoons}\;\text{CO}_2\;-\;\text{Hb}\;-\;\text{H}^{+}\;+\;\text{O}_2(g)\;+\;\text{H}_2\text{O}(l)[/latex]- Write the equilibrium constant expression for this reaction.
- Explain why the production of lactic acid and CO2 in a muscle during exertion stimulates release of O2 from the oxyhemoglobin in the blood passing through the muscle.
- The hydrolysis of the sugar sucrose to the sugars glucose and fructose follows a first-order rate equation for the disappearance of sucrose.
[latex]\text{C}_{12}\text{H}_{22}\text{O}_{11}(aq)\;+\;\text{H}_2\text{O}(l)\;{\longrightarrow}\;\text{C}_6\text{H}_{12}\text{O}_6(aq)\;+\;\text{C}_6\text{H}_{12}\text{O}_6(aq)[/latex]
Rate = k[C12H22O11]
In neutral solution, k = 2.1 × 10−11/s at 27 °C. (As indicated by the rate constant, this is a very slow reaction. In the human body, the rate of this reaction is sped up by a type of catalyst called an enzyme.) (Note: That is not a mistake in the equation—the products of the reaction, glucose and fructose, have the same molecular formulas, C6H12O6, but differ in the arrangement of the atoms in their molecules). The equilibrium constant for the reaction is 1.36 × 105 at 27 °C. What are the concentrations of glucose, fructose, and sucrose after a 0.150 M aqueous solution of sucrose has reached equilibrium? Remember that the activity of a solvent (the effective concentration) is 1. Check Answer: [48]
- The density of trifluoroacetic acid vapour was determined at 118.1 °C and 468.5 torr, and found to be 2.784 g/L. Calculate Kc for the association of the acid shown in the figure below.
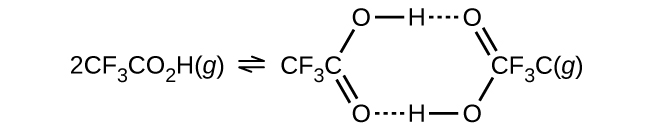
- Liquid N2O3 is dark blue at low temperatures, but the colour fades and becomes greenish at higher temperatures as the compound decomposes to NO and NO2. At 25 °C, a value of KP = 1.91 has been established for this decomposition. If 0.236 moles of N2O3 are placed in a 1.52-L vessel at 25 °C, calculate the equilibrium partial pressures of N2O3(g), NO2(g), and NO(g).
Check Answer: [49] - A 1.00-L vessel at 400 °C contains the following equilibrium concentrations: N2, 1.00 M; H2, 0.50 M; and NH3, 0.25 M. How many moles of hydrogen must be removed from the vessel to increase the concentration of nitrogen to 1.1 M?
- A 0.010 M solution of the weak acid HA has an osmotic pressure (see chapter on solutions and colloids) of 0.293 atm at 25 °C. A 0.010 M solution of the weak acid HB has an osmotic pressure of 0.345 atm under the same conditions.
- Which acid has the larger equilibrium constant for ionization
HA [latex][\text{HA}(aq)\;{\rightleftharpoons}\;\text{A}^{-}(aq)\;+\;\text{H}^{+}(aq)][/latex] or HB [latex][\text{HB}(aq)\;{\rightleftharpoons}\;\text{H}^{+}(aq)\;+\;\text{B}^{-}(aq)][/latex]? - What are the equilibrium constants for the ionization of these acids?
(Hint: Remember that each solution contains three dissolved species: the weak acid (HA or HB), the conjugate base (A− or B−), and the hydrogen ion (H+). Remember that osmotic pressure (like all colligative properties) is related to the total number of solute particles. Specifically for osmotic pressure, those concentrations are described by molarities.) Check Answer: [50]
- Which acid has the larger equilibrium constant for ionization
17.6 Precipitation and Dissolution
- Complete the changes in concentrations for each of the following reactions in the table below:
a. AgI (s) → Ag+ (aq) + I– (aq) x ______ b. CaCO3 (s) → Ca2+ (aq) + CO32- (aq) ______ x c. Mg(OH)2 (s) → Mg2+ (aq) + 2 OH– (aq) x ______ d. Mg3(PO4)2 (s) → 3 Mg2+ (aq) + 2 PO43- (aq) ______ 2x e. Ca5(PO4)3OH (s) → 5 Ca2+ (aq) + 3 PO43- (aq) + OH– (aq) ______ ______ x Check Answer: [51]
- Complete the changes in concentrations for each of the following reactions in the table below:
a. BaSO4 (s) → Ba2+ (aq) + SO42- (aq) x ______ b. Ag2SO4 (s) → 2 Ag+ (aq) + SO42- (aq) ______ x c. Al(OH)3 (s) → Al3+ (aq) + 3 OH– (aq) x ______ d. Pb(OH)Cl (s) → Pb2+ (aq) + OH– (aq) + Cl– (aq) ______ x ______ e. Ca3(AsO4)2 (s) → 3 Ca2+ (aq) + 2 AsO43- (aq) 3x ______ - How do the concentrations of Ag+ and CrO42- in a saturated solution above 1.0 g of solid Ag2CrO4 change when 100 g of solid Ag2CrO4 is added to the system? Explain. Check Answer: [52]
- How do the concentrations of Pb2+ and S2– change when K2S is added to a saturated solution of PbS?
- What additional information do we need to answer the following question: How is the equilibrium of solid silver bromide with a saturated solution of its ions affected when the temperature is raised? Check Answer: [53]
- Which of the following slightly soluble compounds has a solubility greater than that calculated from its solubility product because of hydrolysis of the anion present: CoSO3, CuI, PbCO3, PbCl2, Tl2S, KClO4?
- Which of the following slightly soluble compounds has a solubility greater than that calculated from its solubility product because of hydrolysis of the anion present: AgCl, BaSO4, CaF2, Hg2I2, MnCO3, ZnS, PbS? Check Answer: [54]
- Write the ionic equation for dissolution and the solubility product (Ksp) expression for each of the following slightly soluble ionic compounds:
- PbCl2
- Ag2S
- Sr3(PO4)2
- SrSO4
- Write the ionic equation for the dissolution and the Ksp expression for each of the following slightly soluble ionic compounds:
- LaF3
- CaCO3
- Ag2SO4
- Pb(OH)2
Check Answer: [55]
- The Handbook of Chemistry and Physics gives solubilities of the following compounds in grams per 100 mL of water. Because these compounds are only slightly soluble, assume that the volume does not change on dissolution and calculate the solubility product for each.
- BaSiF6, 0.026 g/100 mL (contains SiF62- ions)
- Ce(IO3)4, 1.5 × 10–2 g/100 mL
- Gd2(SO4)3, 3.98 g/100 mL
- (NH4)2PtBr6, 0.59 g/100 mL (contains PtBr62- ions)
- The Handbook of Chemistry and Physics gives solubilities of the following compounds in grams per 100 mL of water. Because these compounds are only slightly soluble, assume that the volume does not change on dissolution and calculate the solubility product for each.
- BaSeO4, 0.0118 g/100 mL
- Ba(BrO3)2·H2O, 0.30 g/100 mL
- NH4MgAsO4·6H2O, 0.038 g/100 mL
- La2(MoO4)3, 0.00179 g/100 mL
Check Answer: [56]
- Use solubility products and predict which of the following salts is the most soluble, in terms of moles per litre, in pure water: CaF2, Hg2Cl2, PbI2, or Sn(OH)2.
- Assuming that no equilibria other than dissolution are involved, calculate the molar solubility of each of the following from its solubility product:
- KHC4H4O6
- PbI2
- Ag4[Fe(CN)6], a salt containing the Fe(CN)4– ion
- Hg2I2
Check Answer: [57]
- Assuming that no equilibria other than dissolution are involved, calculate the molar solubility of each of the following from its solubility product:
- Ag2SO4
- PbBr2
- AgI
- CaC2O4·H2O
- Assuming that no equilibria other than dissolution are involved, calculate the concentration of all solute species in each of the following solutions of salts in contact with a solution containing a common ion. Show that changes in the initial concentrations of the common ions can be neglected.
- AgCl(s) in 0.025 M NaCl
- CaF2(s) in 0.00133 M KF
- Ag2SO4(s) in 0.500 L of a solution containing 19.50 g of K2SO4
- Zn(OH)2(s) in a solution buffered at a pH of 11.45
Check Answer: [58]
- Assuming that no equilibria other than dissolution are involved, calculate the concentration of all solute species in each of the following solutions of salts in contact with a solution containing a common ion. Show that changes in the initial concentrations of the common ions can be neglected.
- TlCl(s) in 1.250 M HCl
- PbI2(s) in 0.0355 M CaI2
- Ag2CrO4(s) in 0.225 L of a solution containing 0.856 g of K2CrO4
- Cd(OH)2(s) in a solution buffered at a pH of 10.995
- Assuming that no equilibria other than dissolution are involved, calculate the concentration of all solute species in each of the following solutions of salts in contact with a solution containing a common ion. Show that it is not appropriate to neglect the changes in the initial concentrations of the common ions.
- TlCl(s) in 0.025 M TlNO3
- BaF2(s) in 0.0313 M KF
- MgC2O4 in 2.250 L of a solution containing 8.156 g of Mg(NO3)2
- Ca(OH)2(s) in an unbuffered solution initially with a pH of 12.700
Check Answer: [59]
- Calculate the solubility of aluminum hydroxide, Al(OH)3, in a solution buffered at pH 11.00.
- Refer to Appendix K for solubility products for calcium salts. Determine which of the calcium salts listed is most soluble in moles per litre and which is most soluble in grams per litre. Check Answer: [60]
- Most barium compounds are very poisonous; however, barium sulfate is often administered internally as an aid in the X-ray examination of the lower intestinal tract (Figure 17.6c). This use of BaSO4 is possible because of its low solubility. Calculate the molar solubility of BaSO4 and the mass of barium present in 1.00 L of water saturated with BaSO4.
- Public Health Service standards for drinking water set a maximum of 250 mg/L (2.60 × 10–3M) of SO42- because of its cathartic action (it is a laxative). Does natural water that is saturated with CaSO4 (“gyp” water) as a result or passing through soil containing gypsum, CaSO4·2H2O, meet these standards? What is SO42- in such water? Check Answer: [61]
- Perform the following calculations:
- Calculate [Ag+] in a saturated aqueous solution of AgBr.
- What will [Ag+] be when enough KBr has been added to make [Br–] = 0.050 M?
- What will [Br–] be when enough AgNO3 has been added to make [Ag+] = 0.020 M?
- The solubility product of CaSO4·2H2O is 2.4 × 10–5. What mass of this salt will dissolve in 1.0 L of 0.010 M SO42-? Check Answer: [62]
- Assuming that no equilibria other than dissolution are involved, calculate the concentrations of ions in a saturated solution of each of the following (see Appendix K for solubility products).
- TlCl
- BaF2
- Ag2CrO4
- CaC2O4·H2O
- the mineral anglesite, PbSO4
- Assuming that no equilibria other than dissolution are involved, calculate the concentrations of ions in a saturated solution of each of the following (see Appendix K for solubility products):
- AgI
- Ag2SO4
- Mn(OH)2
- Sr(OH)2·8H2O
- the mineral brucite, Mg(OH)2
Check Answer: [63]
- The following concentrations are found in mixtures of ions in equilibrium with slightly soluble solids. From the concentrations given, calculate Ksp for each of the slightly soluble solids indicated:
- AgBr: [Ag+] = 5.7 × 10–7M, [Br–] = 5.7 × 10–7M
- CaCO3: [Ca2+] = 5.3 × 10–3M, [CO32-] = 9.0 × 10–7M
- PbF2: [Pb2+] = 2.1 × 10–3M, [F–] = 4.2 × 10–3M
- Ag2CrO4: [Ag+] = 5.3 × 10–5M, 3.2 × 10–3M
- InF3: [In3+] = 2.3 × 10–3M, [F–] = 7.0 × 10–3M
- The following concentrations are found in mixtures of ions in equilibrium with slightly soluble solids. From the concentrations given, calculate Ksp for each of the slightly soluble solids indicated:
- TlCl: [Tl+] = 1.21 × 10–2M, [Cl–] = 1.2 × 10–2M
- Ce(IO3)4: [Ce4+] = 1.8 × 10–4M, [IO3–] = 2.6 × 10–13M
- Gd2(SO4)3: [Gd3+] = 0.132 M, [SO42-] = 0.198 M
- Ag2SO4: [Ag+] = 2.40 × 10–2M, [SO42-] = 2.05 × 10–2M
- BaSO4: [Ba2+] = 0.500 M, [SO42-] = 2.16 × 10–10M
Check Answer: [64]
- Which of the following compounds precipitates from a solution that has the concentrations indicated? (See Appendix K for Ksp values.)
- KClO4: [K+] = 0.01 M, [ClO4–] = 0.01 M
- K2PtCl6: [K+] = 0.01 M, [PtCl62-] = 0.01 M
- PbI2: [Pb2+] = 0.003 M, [I–] = 1.3 × 10–3M
- Ag2S: [Ag+] = 1 × 10–10M, [S2–] = 1 × 10–13M
- Which of the following compounds precipitates from a solution that has the concentrations indicated? (See Appendix K for Ksp values.)
- CaCO3: [Ca2+] = 0.003 M, [CO32-] = 0.003 M
- Co(OH)2: [Co2+] = 0.01 M, [OH–] = 1 × 10–7M
- CaHPO4: [Ca2+] = 0.01 M, [HPO42-] = 2 × 10–6M
- Pb3(PO4)2: [Pb2+] = 0.01 M, [PO43-] = 1 × 10–13M
Check Answer: [65]
- Calculate the concentration of Tl+ when TlCl just begins to precipitate from a solution that is 0.0250 M in Cl–.
- Calculate the concentration of sulfate ion when BaSO4 just begins to precipitate from a solution that is 0.0758 M in Ba2+. Check Answer: [66]
- Calculate the concentration of Sr2+ when SrF2 starts to precipitate from a solution that is 0.0025 M in F–.
- Calculate the concentration of PO43- when Ag3PO4 starts to precipitate from a solution that is 0.0125 M in Ag+. Check Answer: [67]
- Calculate the concentration of F– required to begin precipitation of CaF2 in a solution that is 0.010 M in Ca2+.
- Calculate the concentration of Ag+ required to begin precipitation of Ag2CO3 in a solution that is 2.50 × 10–6M in CO32-. Check Answer: [68]
- What [Ag+] is required to reduce [CO32-] to 8.2 × 10–4M by precipitation of Ag2CO3?
- What [F–] is required to reduce [Ca2+] to 1.0 × 10–4M by precipitation of CaF2? Check Answer: [69]
- A volume of 0.800 L of a 2 × 10–4–M Ba(NO3)2 solution is added to 0.200 L of 5 × 10–4M Li2SO4. Does BaSO4 precipitate? Explain your answer.
- Perform these calculations for nickel(II) carbonate.
- With what volume of water must a precipitate containing NiCO3 be washed to dissolve 0.100 g of this compound? Assume that the wash water becomes saturated with NiCO3 (Ksp = 1.36 × 10–7).
- If the NiCO3 were a contaminant in a sample of CoCO3 (Ksp = 1.0 × 10–12), what mass of CoCO3 would have been lost? Keep in mind that both NiCO3 and CoCO3 dissolve in the same solution.
Check Answer: [70]
- Iron concentrations greater than 5.4 × 10–6M in water used for laundry purposes can cause staining. What [OH–] is required to reduce [Fe2+] to this level by precipitation of Fe(OH)2?
- A solution is 0.010 M in both Cu2+ and Cd2+. What percentage of Cd2+ remains in the solution when 99.9% of the Cu2+ has been precipitated as CuS by adding sulfide? Check Answer: [71]
- A solution is 0.15 M in both Pb2+ and Ag+. If Cl– is added to this solution, what is [Ag+] when PbCl2 begins to precipitate?
- What reagent might be used to separate the ions in each of the following mixtures, which are 0.1 M with respect to each ion? In some cases it may be necessary to control the pH. (Hint: Consider the Ksp values given in Appendix K.)
- Hg22+ and Cu2+
- SO42- and Cl–
- Hg2+ and Co2+
- Zn2+ and Sr2+
- Ba2+ and Mg2+
- CO32- and OH–
Check Answer: [72]
- A solution contains 1.0 × 10–5 mol of KBr and 0.10 mol of KCl per litre. AgNO3 is gradually added to this solution. Which forms first, solid AgBr or solid AgCl?
- A solution contains 1.0 × 10–2 mol of KI and 0.10 mol of KCl per litre. AgNO3 is gradually added to this solution. Which forms first, solid AgI or solid AgCl? Check Answer: [73]
- The calcium ions in human blood serum are necessary for coagulation. Potassium oxalate, K2C2O4, is used as an anticoagulant when a blood sample is drawn for laboratory tests because it removes the calcium as a precipitate of CaC2O4·H2O. It is necessary to remove all but 1.0% of the Ca2+ in serum in order to prevent coagulation. If normal blood serum with a buffered pH of 7.40 contains 9.5 mg of Ca2+ per 100 mL of serum, what mass of K2C2O4 is required to prevent the coagulation of a 10 mL blood sample that is 55% serum by volume? (All volumes are accurate to two significant figures. Note that the volume of serum in a 10-mL blood sample is 5.5 mL. Assume that the Ksp value for CaC2O4 in serum is the same as in water.)
- About 50% of urinary calculi (kidney stones) consist of calcium phosphate, Ca3(PO4)2. The normal mid range calcium content excreted in the urine is 0.10 g of Ca2+ per day. The normal mid range amount of urine passed may be taken as 1.4 L per day. What is the maximum concentration of phosphate ion that urine can contain before a calculus begins to form? Check Answer: [74]
- The pH of normal urine is 6.30, and the total phosphate concentration [PO43-] + [HPO42-] + [H2PO4–] + [H3PO4] is 0.020 M. What is the minimum concentration of Ca2+ necessary to induce kidney stone formation?
- Magnesium metal (a component of alloys used in aircraft and a reducing agent used in the production of uranium, titanium, and other active metals) is isolated from sea water by the following sequence of reactions:
[latex]\text{Mg}^{2+}(aq)\;+\;\text{Ca(OH)}_2(aq)\;{\longrightarrow}\;\text{Mg(OH)}_2(s)\;+\;\text{Ca}^{2+}(aq)[/latex]
[latex]\text{Mg(OH)}_2(s)\;+\;2\text{ HCl}(aq)\;{\longrightarrow}\;\text{MgCl}_2(s)\;+\;2\text{ H}_2\text{O}(l)[/latex]
[latex]\text{MgCl}_2(l)\;{\xrightarrow{\text{electrolysis}}}\;\text{Mg}(s)\;+\;\text{Cl}_2(g)[/latex]
Sea water has a density of 1.026 g/cm3 and contains 1272 parts per million of magnesium as Mg2+(aq) by mass. What mass, in kilograms, of Ca(OH)2 is required to precipitate 99.9% of the magnesium in 1.00 × 103 L of sea water? Check Answer: [75] - Hydrogen sulfide is bubbled into a solution that is 0.10 M in both Pb2+ and Fe2+ and 0.30 M in HCl. After the solution has come to equilibrium it is saturated with H2S ([H2S] = 0.10 M). What concentrations of Pb2+ and Fe2+ remain in the solution? For a saturated solution of H2S we can use the equilibrium:
[latex]\text{H}_2\text{S}(aq)\;+\;2\text{ H}_2\text{O}(l)\;{\leftrightharpoons}\;2\text{ H}_3\text{O}^{+}(aq)\;+\;\text{S}^{2-}(aq)\;\;\;\;\;\;\;K = 1.0\;\times\;10^{-26}[/latex]
(Hint: The [H3O+] changes as metal sulfides precipitate.)
- Perform the following calculations involving concentrations of iodate ions:
- The iodate ion concentration of a saturated solution of La(IO3)3 was found to be 3.1 × 10–3 mol/L. Find the Ksp.
- Find the concentration of iodate ions in a saturated solution of Cu(IO3)2 (Ksp = 7.4 × 10–8).
Check Answer: [76]
- Calculate the molar solubility of AgBr in 0.035 M NaBr (Ksp = 5 × 10–13).
- How many grams of Pb(OH)2 will dissolve in 500 mL of a 0.050-M PbCl2 solution (Ksp = 1.2 × 10–15)? Check Answer: [77]
- How many grams of Milk of Magnesia, Mg(OH)2 (s) (58.3 g/mol), would be soluble in 200 mL of water. Ksp = 7.1 × 10–12. Include the ionic reaction and the expression for Ksp in your answer. (Kw = 1 × 10–14 = [H3O+][OH–])
- Two hypothetical salts, LM2 and LQ, have the same molar solubility in H2O. If Ksp for LM2 is 3.20 × 10–5, what is the Ksp value for LQ? Check Answer: [78]
- Which of the following carbonates will form first? Which of the following will form last? Explain.
- [latex]\text{MgCO}_3\;\;\;\;\;\;\;K_{\text{sp}} = 3.5\;\times\;10^{-8}[/latex]
- [latex]\text{CaCO}_3\;\;\;\;\;\;\;K_{\text{sp}} = 4.2\;\times\;10^{-7}[/latex]
- [latex]\text{SrCO}_3\;\;\;\;\;\;\;K_{\text{sp}} = 3.9\;\times\;10^{-9}[/latex]
- [latex]\text{BaCO}_3\;\;\;\;\;\;\;K_{\text{sp}} = 4.4\;\times\;10^{-5}[/latex]
- [latex]\text{MnCO}_3\;\;\;\;\;\;\;K_{\text{sp}} = 5.1\;\times\;10^{-9}[/latex]
Check Answer: [79]
- How many grams of Zn(CN)2(s) (117.44 g/mol) would be soluble in 100 mL of H2O? Include the balanced reaction and the expression for Ksp in your answer. The Ksp value for Zn(CN)2(s) is 3.0 × 10–16.
17.7 Relative Strengths of Acids and Bases
- Explain why the neutralization reaction of a strong acid and a weak base gives a weakly acidic solution.
- Explain why the neutralization reaction of a weak acid and a strong base gives a weakly basic solution. Check Answer: [80]
- Use this list of important industrial compounds (and Figure 17.7c) to answer the following questions regarding: CaO, Ca(OH)2, CH3CO2H, CO2, HCl, H2CO3, HF, HNO2, HNO3, H3PO4, H2SO4, NH3, NaOH, Na2CO3.
- Identify the strong Brønsted-Lowry acids and strong Brønsted-Lowry bases.
- List those compounds in (a) that can behave as Brønsted-Lowry acids with strengths lying between those of H3O+ and H2O.
- List those compounds in (a) that can behave as Brønsted-Lowry bases with strengths lying between those of H2O and OH−.
- The odour of vinegar is due to the presence of acetic acid, CH3CO2H, a weak acid. List, in order of descending concentration, all of the ionic and molecular species present in a 1-M aqueous solution of this acid. Check Answer: [81]
- Household ammonia is a solution of the weak base NH3 in water. List, in order of descending concentration, all of the ionic and molecular species present in a 1-M aqueous solution of this base.
- Explain why the ionization constant, Ka, for H2SO4 is larger than the ionization constant for H2SO3. Check Answer: [82]
- Explain why the ionization constant, Ka, for HI is larger than the ionization constant for HF.
- Gastric juice, the digestive fluid produced in the stomach, contains hydrochloric acid, HCl. Milk of Magnesia, a suspension of solid Mg(OH)2 in an aqueous medium, is sometimes used to neutralize excess stomach acid. Write a complete balanced equation for the neutralization reaction, and identify the conjugate acid-base pairs. Check Answer: [83]
- Nitric acid reacts with insoluble copper(II) oxide to form soluble copper(II) nitrate, Cu(NO3)2, a compound that has been used to prevent the growth of algae in swimming pools. Write the balanced chemical equation for the reaction of an aqueous solution of HNO3 with CuO.
- What is the ionization constant at 25 °C for the weak acid CH3NH3+, the conjugate acid of the weak base CH3NH2, Kb = 4.4 × 10−4. Check Answer: [84]
- What is the ionization constant at 25 °C for the weak acid (CH3)2NH2+, the conjugate acid of the weak base (CH3)2NH, Kb = 5.9 × 10−4?
- Which base, CH3NH2 or (CH3)2NH, is the stronger base? Which conjugate acid, (CH3)2NH2+ or (CH3)2NH, is the stronger acid? Check Answer: [85]
- Which is the stronger acid, NH4+ or HBrO?
- Which is the stronger base, (CH3)3N or H2BO3–? Check Answer: [86]
- Predict which acid in each of the following pairs is the stronger and explain your reasoning for each.
- H2O or HF
- B(OH)3 or Al(OH)3
- HSO3– or HSO4–
- NH3 or H2S
- H2O or H2Te
- Predict which compound in each of the following pairs of compounds is more acidic and explain your reasoning for each.
- HSO4– or HSeO4–
- NH3 or H2O
- PH3 or HI
- NH3 or PH3
- H2S or HBr
Check Answer: [87]
- Rank the compounds in each of the following groups in order of increasing acidity or basicity, as indicated, and explain the order you assign.
- acidity: HCl, HBr, HI
- basicity: H2O, OH−, H−, Cl−
- basicity: Mg(OH)2, Si(OH)4, ClO3(OH) (Hint: Formula could also be written as HClO4).
- acidity: HF, H2O, NH3, CH4
- Rank the compounds in each of the following groups in order of increasing acidity or basicity, as indicated, and explain the order you assign.
- acidity: NaHSO3, NaHSeO3, NaHSO4
- basicity: BrO2–, ClO2–, IO2–
- acidity: HOCl, HOBr, HOI
- acidity: HOCl, HOClO, HOClO2, HOClO3
- basicity: NH2–, HS−, HTe−, PH2–
- basicity: BrO−, BrO2–, BrO3–, BrO4–
Check Answer: [88]</li>
- Both HF and HCN ionize in water to a limited extent. Which of the conjugate bases, F− or CN−, is the stronger base? See Table 17.7b.
- The active ingredient formed by aspirin in the body is salicylic acid, C6H4OH(CO2H). The carboxyl group (−CO2H) acts as a weak acid. The phenol group (an OH group bonded to an aromatic ring) also acts as an acid but a much weaker acid. List, in order of descending concentration, all of the ionic and molecular species present in a 0.001-M aqueous solution of C6H4OH(CO2H). Check Answer: [89]
- What do we represent when we write:
[latex]\text{CH}_3\text{CO}_2\text{H}(aq)\;+\;\text{H}_2\text{O}(l)\;{\rightleftharpoons}\;\text{H}_3\text{O}^{+}(aq)\;+\;\text{CH}_3\text{CO}_2^{\;\;-}(aq)[/latex]? - Explain why equilibrium calculations are not necessary to determine ionic concentrations in solutions of certain strong electrolytes such as NaOH and HCl. Under what conditions are equilibrium calculations necessary as part of the determination of the concentrations of all ions of some other strong electrolytes in solution? Check Answer: [90]
- Are the concentrations of hydronium ion and hydroxide ion in a solution of an acid or a base in water directly proportional or inversely proportional? Explain your answer.
- What two common assumptions can simplify calculation of equilibrium concentrations in a solution of a weak acid? Check Answer: [91]
- What two common assumptions can simplify calculation of equilibrium concentrations in a solution of a weak base?
- Which of the following will increase the percent of NH3 that is converted to the ammonium ion in water (Hint: Use LeChâtelier’s principle.)?
- addition of NaOH
- addition of HCl
- addition of NH4Cl
Check Answer: [92]
- Which of the following will increase the percent of HF that is converted to the fluoride ion in water?
- addition of NaOH
- addition of HCl
- addition of NaF
- What is the effect on the concentrations of NO2−, HNO2, and OH− when the following are added to a solution of KNO2 in water:
- HCl
- HNO2
- NaOH
- NaCl
- KNO
The equation for the equilibrium is:[latex]\text{NO}_2^{\;\;-}(aq)\;+\;\text{H}_2\text{O}(l)\;{\rightleftharpoons}\;\text{HNO}_2(aq)\;+\;\text{OH}^{-}(aq)[/latex]
Check Answer: [93]
- What is the effect on the concentration of hydrofluoric acid, hydronium ion, and fluoride ion when the following are added to separate solutions of hydrofluoric acid?
- HCl
- KF
- NaCl
- KOH
- HF
The equation for the equilibrium is:[latex]\text{HF}(aq)\;+\;\text{H}_2\text{O}(l)\;{\rightleftharpoons}\;\text{H}_3\text{O}^{+}(aq)\;+\;\text{F}^{-}(aq)[/latex]
- Why is the hydronium ion concentration in a solution that is 0.10 M in HCl and 0.10 M in HCOOH determined by the concentration of HCl? Check Answer: [94]
- From the equilibrium concentrations given, calculate Ka for each of the weak acids and Kb for each of the weak bases.
- CH3CO2H: [H3O+] = 1.34 × 10−3M;
[CH3CO2-] = 1.34 × 10−3M;
[CH3CO2H] = 9.866 × 10−2M; -
ClO−: [OH−] = 4.0 × 10−4M;
[HClO] = 2.38 × 10−5M;
[ClO−] = 0.273 M; -
HCO2H: [HCO2H] = 0.524 M;
[H3O+] = 9.8 × 10−3M;
[HCO2-] = 9.8 × 10−3M; -
C6H5NH3+: [C6H5NH3+] = 0.233 M;
[C6H5NH2] = 2.3 × 10−3M;
[H3O+] = 2.3 × 10−3M
- CH3CO2H: [H3O+] = 1.34 × 10−3M;
- From the equilibrium concentrations given, calculate Ka for each of the weak acids and Kb for each of the weak bases.
- NH3: [OH−] = 3.1 × 10−3M;
[NH4+] = 3.1 × 10−3M;
[NH3] = 0.533 M; -
HNO2: [H3O+] = 0.011 M;
[NO2-] = 0.0438 M;
[HNO2] = 1.07 M; -
(CH3)3N: [(CH3)3N] = 0.25 M;
[(CH3)3NH+] = 4.3 × 10−3M;
[OH−] = 4.3 × 10−3M; -
NH4+: [NH4+] = 0.100 M;
[NH3] = 7.5 × 10−6M;
[H3O+] = 7.5 × 10−6M
Check Answer: [95]
- NH3: [OH−] = 3.1 × 10−3M;
- Determine Kb for the nitrite ion, NO2-. In a 0.10-M solution this base is 0.0015% ionized.
- Determine Ka for hydrogen sulfate ion, HSO4-. In a 0.10-M solution the acid is 29% ionized. Check Answer: [96]
- Calculate the ionization constant for each of the following acids or bases from the ionization constant of its conjugate base or conjugate acid:
- [latex]\text{F}^{-}[/latex]
- [latex]\text{NH}_4^{\;+}[/latex]
- [latex]\text{AsO}_4^{\;3-}[/latex]
- [latex](\text{CH}_3)_2\text{NH}_2^{\;+}[/latex]
- [latex]\text{NO}_2^{\;-}[/latex]
- [latex]\text{HC}_2\text{O}_4^{\;-}[/latex] (as a base)
- Calculate the ionization constant for each of the following acids or bases from the ionization constant of its conjugate base or conjugate acid:
- [latex]\text{HTe}^{-}[/latex] (as a base)
- [latex](\text{CH}_3)_3\text{NH}^{+}[/latex]
- [latex]\text{HAsO}_4^{\;3-}[/latex] (as a base)
- [latex]\text{HO}_2^{\;-}[/latex] (as a base)
- [latex]\text{C}_6\text{H}_5\text{NH}_3^{\;+}[/latex]
- [latex]\text{HSO}_3^{\;-}[/latex] (as a base)
Check Answer: [97]
- For which of the following solutions must we consider the ionization of water when calculating the pH or pOH?
- 3 × 10−8M HNO3
- 0.10 g HCl in 1.0 L of solution
- 0.00080 g NaOH in 0.50 L of solution
- 1 × 10−7M Ca(OH)2
- 0.0245 M KNO3
- Even though both NH3 and C6H5NH2 are weak bases, NH3 is a much stronger acid than C6H5NH2. Which of the following is correct at equilibrium for a solution that is initially 0.10 M in NH3 and 0.10 M in C6H5NH2?
- [latex][\text{OH}^{-}] = [\text{NH}_4^{\;+}][/latex]
- [latex][\text{NH}_4^{\;+}] = [\text{C}_6\text{H}_5\text{NH}_3^{\;+}][/latex]
- [latex][\text{OH}^{-}] = [\text{C}_6\text{H}_5\text{NH}_3^{\;+}][/latex]
- [latex][\text{NH}_3] = [\text{C}_6\text{H}_5\text{NH}_2][/latex]
- both a and b are correct
Check Answer: [98]
- Calculate the equilibrium concentration of the nonionized acids and all ions in a solution that is 0.25 M in HCO2H and 0.10 M in HClO.
- Calculate the equilibrium concentration of the nonionized acids and all ions in a solution that is 0.134 M in HNO2 and 0.120 M in HBrO. Check Answer: [99]
- Calculate the equilibrium concentration of the nonionized bases and all ions in a solution that is 0.25 M in CH3NH2 and 0.10 M in C5H5N (Kb = 1.7 × 10−9).
- Calculate the equilibrium concentration of the nonionized bases and all ions in a solution that is 0.115 M in NH3 and 0.100 M in C6H5NH2. Check Answer: [100]
- Using the Ka value of 1.4 × 10−5, place Al(H2O)63+ in the correct location in Figure 17.7c.
- Calculate the concentration of all solute species in each of the following solutions of acids or bases. Assume that the ionization of water can be neglected, and show that the change in the initial concentrations can be neglected. Ionization constants can be found in Appendix I and Appendix J.
- 0.0092 M HClO, a weak acid
- 0.0784 M C6H5NH2, a weak base
- 0.0810 M HCN, a weak acid
- 0.11 M (CH3)3N, a weak base
- 0.120 M FeH2O62+ a weak acid, Ka = 1.6 × 10−7
Check Answer: [101]
- Propionic acid, C2H5CO2H (Ka = 1.34 × 10−5), is used in the manufacture of calcium propionate, a food preservative. What is the hydronium ion concentration in a 0.698-M solution of C2H5CO2H?
- White vinegar is a 5.0% by mass solution of acetic acid in water. If the density of white vinegar is 1.007 g/cm3, what is the pH? Check Answer: [102]
- The ionization constant of lactic acid, CH3CH(OH)CO2H, an acid found in the blood after strenuous exercise, is 1.36 × 10−4. If 20.0 g of lactic acid is used to make a solution with a volume of 1.00 L, what is the concentration of hydronium ion in the solution?
- Nicotine, C10H14N2, is a base that will accept two protons (K1 = 7 × 10−7, K2 = 1.4 × 10−11). What is the concentration of each species present in a 0.050-M solution of nicotine? Check Answer: [103]
- The pH of a 0.20-M solution of HF is 1.92. Determine Ka for HF from these data.
- The pH of a 0.15-M solution of HSO4- is 1.43. Determine Ka for HSO4- from these data. Check Answer: [104]
- The pH of a 0.10-M solution of caffeine is 11.16. Determine Kb for caffeine from these data:
[latex]\text{C}_8\text{H}_{10}\text{N}_4\text{O}_2(aq)\;+\;\text{H}_2\text{O}(l)\;{\leftrightharpoons}\;\text{C}_8\text{H}_{10}\text{N}_4\text{O}_2\text{H}^{+}(aq)\;+\;\text{OH}^{-}(aq)[/latex] - The pH of a solution of household ammonia, a 0.950 M solution of NH3, is 11.612. Determine Kb for NH3 from these data. Check Answer: [105]
17.8 Real World Examples of Equilibria
- A saturated solution of a slightly soluble electrolyte in contact with some of the solid electrolyte is said to be a system in equilibrium. Explain. Why is such a system called a heterogeneous equilibrium?
- Calculate the equilibrium concentration of Ni2+ in a 1.0-M solution [Ni(NH3)6](NO3)2. Check Answer: [106]
- Calculate the equilibrium concentration of Zn2+ in a 0.30-M solution of Zn(CN)42-.
- Calculate the equilibrium concentration of Cu2+ in a solution initially with 0.050 M Cu2+ and 1.00 M NH3. Check Answer: [107]
- Calculate the equilibrium concentration of Zn2+ in a solution initially with 0.150 M Zn2+ and 2.50 M CN–.
- Calculate the Fe3+ equilibrium concentration when 0.0888 mole of K3[Fe(CN)6] is added to a solution with 0.0.00010 M CN–. Check Answer: [108]
- Calculate the Co2+ equilibrium concentration when 0.100 mole of [Co(NH3)6](NO3)2 is added to a solution with 0.025 M NH3. Assume the volume is 1.00 L.
- The equilibrium constant for the reaction [latex]\text{Hg}^{2+}(aq)\;+\;2\text{ Cl}^{-}(aq)\;{\leftrightharpoons}\;\text{HgCl}_2(aq)[/latex] is 1.6 × 1013. Is HgCl2 a strong electrolyte or a weak electrolyte? What are the concentrations of Hg2+ and Cl– in a 0.015-M solution of HgCl2? Check Answer: [109]
- Calculate the molar solubility of Sn(OH)2 in a buffer solution containing equal concentrations of NH3 and NH4+.
- Calculate the molar solubility of Al(OH)3 in a buffer solution with 0.100 M NH3 and 0.400 M NH4+. Check Answer: [110]
- What is the molar solubility of CaF2 in a 0.100-M solution of HF? Ka for HF = 7.2 × 10–4.
- What is the molar solubility of BaSO4 in a 0.250-M solution of NaHSO4? Ka for HSO4- = 1.2 × 10–2. Check Answer: [111]
- What is the molar solubility of Tl(OH)3 in a 0.10-M solution of NH3?
- What is the molar solubility of Pb(OH)2 in a 0.138-M solution of CH3NH2? Check Answer: [112]
- A solution of 0.075 M CoBr2 is saturated with H2S ([H2S] = 0.10 M). What is the minimum pH at which CoS begins to precipitate?
[latex]\text{CoS}(s)\;{\leftrightharpoons}\;\text{Co}^{2+}(aq)\;+\;\text{S}^{2-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = 4.5\;\times\;10^{-27}[/latex]
[latex]\text{H}_2\text{S}(aq)\;+\;2\text{ H}_2\text{O}(l)\;{\leftrightharpoons}\;2\text{ H}_3\text{O}^{+}(aq)\;+\;\text{S}^{2-}(aq)\;\;\;\;\;\;\;K = 1.0\;\times\;10^{-26}[/latex] - A 0.125-M solution of Mn(NO3)2 is saturated with H2S ([H2S] = 0.10 M). At what pH does MnS begin to precipitate?
[latex]\text{MnS}(s)\;{\rightleftharpoons}\;\text{Mn}^{2+}(aq)\;+\;\text{S}^{2-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = 4.3\;\times\;10^{-22}[/latex]
[latex]\text{H}_2\text{S}(aq)\;+\;2\text{ H}_2\text{O}(l)\;{\rightleftharpoons}\;2\text{ H}_3\text{O}^{+}(aq)\;+\;\text{S}^{2-}(aq)\;\;\;\;\;\;\;K = 1.0\;\times\;10^{-26}[/latex]
Check Answer: [113] - Calculate the molar solubility of BaF2 in a buffer solution containing 0.20 M HF and 0.20 M NaF.
- Calculate the molar solubility of CdCO3 in a buffer solution containing 0.115 M Na2CO3 and 0.120 M NaHCO3 Check Answer: [114]
- To a 0.10-M solution of Pb(NO3)2 is added enough HF(g) to make [HF] = 0.10 M.
- Does PbF2 precipitate from this solution? Show the calculations that support your conclusion.
- What is the minimum pH at which PbF2 precipitates?
- Calculate the concentration of Cd2+ resulting from the dissolution of CdCO3 in a solution that is 0.010 M in H2CO3. Check Answer: [115]
- Both AgCl and AgI dissolve in NH3.
- What mass of AgI dissolves in 1.0 L of 1.0 M NH3?
- What mass of AgCl dissolves in 1.0 L of 1.0 M NH3?
- Calculate the volume of 1.50 M CH3CO2H required to dissolve a precipitate composed of 350 mg each of CaCO3, SrCO3, and BaCO3. Check Answer: [116]
- Even though Ca(OH)2 is an inexpensive base, its limited solubility restricts its use. What is the pH of a saturated solution of Ca(OH)2?
- What mass of NaCN must be added to 1 L of 0.010 M Mg(NO3)2 in order to produce the first trace of Mg(OH)2? Check Answer: [117]
- Magnesium hydroxide and magnesium citrate function as mild laxatives when they reach the small intestine. Why do magnesium hydroxide and magnesium citrate, two very different substances, have the same effect in your small intestine. (Hint: The contents of the small intestine are basic.)
- The following question is taken from a Chemistry Advanced Placement Examination and is used with the permission of the Educational Testing Service.
Solve the following problem:
[latex]\text{MgF}_2(s)\;{\rightleftharpoons}\;\text{Mg}^{2+}(aq)\;+\;2\text{ F}^{-}(aq)[/latex]In a saturated solution of MgF2 at 18 °C, the concentration of Mg2+ is 1.21 × 10–3M. The equilibrium is represented by the preceding equation.
- Write the expression for the solubility-product constant, Ksp, and calculate its value at 18 °C.
- Calculate the equilibrium concentration of Mg2+ in 1.000 L of saturated MgF2 solution at 18 °C to which 0.100 mol of solid KF has been added. The KF dissolves completely. Assume the volume change is negligible.
- Predict whether a precipitate of MgF2 will form when 100.0 mL of a 3.00 × 10–3-M solution of Mg(NO3)2 is mixed with 200.0 mL of a 2.00 × 10–3-M solution of NaF at 18 °C. Show the calculations to support your prediction.
- At 27 °C the concentration of Mg2+ in a saturated solution of MgF2 is 1.17 × 10–3M. Is the dissolving of MgF2 in water an endothermic or an exothermic process? Give an explanation to support your conclusion.
Check Answer: [118]
- Which of the following compounds, when dissolved in a 0.01-M solution of HClO4, has a solubility greater than in pure water: CuCl, CaCO3, MnS, PbBr2, CaF2? Explain your answer.
- Which of the following compounds, when dissolved in a 0.01-M solution of HClO4, has a solubility greater than in pure water: AgBr, BaF2, Ca3(PO4)3, ZnS, PbI2? Explain your answer. Check Answer: [119]
- What is the effect on the amount of solid Mg(OH)2 that dissolves and the concentrations of Mg2+ and OH– when each of the following are added to a mixture of solid Mg(OH)2 and water at equilibrium?
- MgCl2
- KOH
- HClO4
- NaNO3
- Mg(OH)2
- What is the effect on the amount of CaHPO4 that dissolves and the concentrations of Ca2+ and HPO4- when each of the following are added to a mixture of solid CaHPO4 and water at equilibrium?
- CaCl2
- HCl
- KClO4
- NaOH
- CaHPO4
Check Answer: [120]
- Identify all chemical species present in an aqueous solution of Ca3(PO4)2 and list these species in decreasing order of their concentrations. (Hint: Remember that the PO43- ion is a weak base.)
- A volume of 50 mL of 1.8 M NH3 is mixed with an equal volume of a solution containing 0.95 g of MgCl2. What mass of NH4Cl must be added to the resulting solution to prevent the precipitation of Mg(OH)2? Check Answer: [121]
Attribution & References
- The instantaneous rate is the rate of a reaction at any particular point in time, a period of time that is so short that the concentrations of reactants and products change by a negligible amount. The initial rate is the instantaneous rate of reaction as it starts (as product just begins to form). Average rate is the average of the instantaneous rates over a time period. ↵
- [latex]\text{rate} = +\frac{1}{2}\;\frac{{\Delta}[\text{CIF}_3]}{{\Delta}t} = -\frac{{\Delta}[\text{Cl}_2]}{{\Delta}t} = -\frac{1}{3}\;\frac{{\Delta}[\text{F}_2]}{{\Delta}t}[/latex] ↵
- (a) average rate, 0 − 10 s = 0.0375 mol L−1 s−1; average rate, 12 − 18 s = 0.0225 mol L−1 s−1; (b) instantaneous rate, 15 s = 0.0500 mol L−1 s−1; (c) average rate for B formation = 0.0188 mol L−1 s−1; instantaneous rate for B formation = 0.0250 mol L−1 s−1 ↵
- The reaction can proceed in both the forward and reverse directions. ↵
- When a system has reached equilibrium, no further changes in the reactant and product concentrations occur; the reactions continue to occur, but at equivalent rates. ↵
- The concept of equilibrium does not imply equal concentrations, though it is possible. ↵
- Equilibrium cannot be established between the liquid and the gas phase if the top is removed from the bottle because the system is not closed; one of the components of the equilibrium, the Br2 vapor, would escape from the bottle until all liquid disappeared. Thus, more liquid would evaporate than can condense back from the gas phase to the liquid phase. ↵
- (a) Kc = [Ag+][Cl−] < 1. AgCl is insoluble; thus, the concentrations of ions are much less than 1 M; (b) [latex]K_c = \frac{1}{[\text{Pb}^{2+}][\text{Cl}^{-}]^2}[/latex] > 1 because PbCl2 is insoluble and formation of the solid will reduce the concentration of ions to a low level (<1 M). ↵
- Since [latex]K_c = \frac{[\text{C}_6\text{H}_6]}{[\text{C}_2\text{H}_2]^3}[/latex], a value of Kc ≈ 10 means that C6H6 predominates over C2H2. In such a case, the reaction would be commercially feasible if the rate to equilibrium is suitable. ↵
- Kc > 1 ↵
- (a) [latex]Q_c = \frac{[\text{CH}_3\text{Cl}][\text{HCl}]}{[\text{CH}_4][\text{Cl}_2]}[/latex]; (b) [latex]Q_c = \frac{[\text{NO}]^2}{[\text{N}_2][\text{O}_2]}[/latex]; (c) [latex]Q_c = \frac{[\text{SO}_3]^2}{[\text{SO}_2]^2[\text{O}_2]}[/latex]; (d) [latex]Q_c = [\text{SO}_2][/latex]; (e) [latex]Q_c = \frac{1}{[\text{P}_4][\text{O}_2]^5}[/latex]; (f) [latex]Q_c = \frac{[\text{Br}]^2}{[\text{Br}_2]}[/latex]; (g) [latex]Q_c = \frac{[\text{CO}_2]}{[\text{CH}_4][\text{O}_2]^2}[/latex]; (h) [latex]Q_c = [\text{H}_2\text{O}]^5[/latex] ↵
- (a) Qc 25 proceeds left; (b) QP 0.22 proceeds right; (c) Qc undefined proceeds left; (d) QP 1.00 proceeds right; (e) QP 0 proceeds right; (f) Qc 4 proceeds left ↵
- The system will shift toward the reactants to reach equilibrium. ↵
- (a) KP = 1.6 × 10−4; (b) KP = 50.2; (c) Kc = 5.31 × 10−39; (d) Kc = 4.60 × 10−3 ↵
- [latex]K_P = P_{\text{H}_2\text{O}} = 0.042[/latex]. ↵
- [latex]Q_c = \frac{[\text{NH}_4^{\;\;+}][\text{OH}^{-}]}{[\text{NH}_3]}[/latex] ↵
- The amount of CaCO3 must be so small that [latex]P_{\text{CO}_2}[/latex] is less than KP when the CaCO3 has completely decomposed. In other words, the starting amount of CaCO3 cannot completely generate the full [latex]P_{\text{CO}_2}[/latex] required for equilibrium. ↵
- The change in enthalpy may be used. If the reaction is exothermic, the heat produced can be thought of as a product. If the reaction is endothermic the heat added can be thought of as a reactant. Additional heat would shift an exothermic reaction back to the reactants but would shift an endothermic reaction to the products. Cooling an exothermic reaction causes the reaction to shift toward the product side; cooling an endothermic reaction would cause it to shift to the reactants' side. ↵
- No, it is not at equilibrium. Because the system is not confined, products continuously escape from the region of the flame; reactants are also added continuously from the burner and surrounding atmosphere. ↵
- Add N2; add H2; decrease the container volume; heat the mixture. ↵
- (a) [latex]K_c = \frac{[\text{CO}][\text{H}_2]}{[\text{H}_2\text{O}]}[/latex]; (b) [H2O] no change, [CO] no change, [H2] no change; (c) [H2O] decreases, [CO] decreases, [H2] decreases; (d) [H2O] increases, [CO] increases, [H2] decreases; (f) [H2O] decreases, [CO] increases, [H2] increases. In (b), (c), (d), and (e), the mass of carbon will change, but its concentration (activity) will not change. ↵
- Only (b) ↵
- Add NaCl or some other salt that produces Cl− to the solution. Cooling the solution forces the equilibrium to the right, precipitating more AgCl(s). ↵
- (a) ↵
- [latex]K_c = \frac{[\text{C}]^2}{[\text{A}][\text{B}]^2}[/latex]. [A] = 0.1 M, [B] = 0.1 M, [C] = 1 M; and [A] = 0.01, [B] = 0.250, [C] = 0.791. ↵
- Kc = 6.00 × 10−2 ↵
- Kc = 0.50 ↵
- The equilibrium equation is KP = 1.9 × 103 ↵
- KP = 3.06 ↵
- (a) −2x, 2x, −0.250 M, 0.250 M; (b) 4x, −2x, −6x, 0.32 M, −0.16 M, −0.48 M; (c) −2x, 3x, −50 torr, 75 torr; (d) x, − x, −3x, 5 atm, −5 atm, −15 atm; (e) x, 1.03 × 10−4M; (f) x, 0.1 atm. ↵
- Activities of pure crystalline solids equal 1 and are constant; however, the mass of Ni does change. ↵
- [NH3] = 9.1 × 10−2M ↵
- PBrCl = 4.9 × 10−2 atm ↵
- [CO] = 2.0 × 10−4M ↵
- [latex]P_{\text{H}_2\text{O}} = 3.64\;\times\;10^{-3}\;\text{atm}[/latex] ↵
- Calculate Q based on the calculated concentrations and see if it is equal to Kc. Because Q does equal 4.32, the system must be at equilibrium. ↵
- (a) [NO2] = 1.17 × 10−3M ; [N2O4] = 0.128 M (b) Percent error [latex]= \frac{5.87\;\times\;10^{-4}}{0.129}\;\times\;100\% = 0.455\%[/latex]. The change in concentration of N2O4 is far less than the 5% maximum allowed. ↵
- (a) [H2S] = 0.810 atm ; [H2] = 0.014 atm ; [S2] = 0.0072 atm (b) The 2x is dropped from the equilibrium calculation because 0.014 is negligible when subtracted from 0.824. The percent error associated with ignoring 2x is [latex]\frac{0.014}{0.824}\;\times\;100\% = 1.7\%[/latex], which is less than allowed by the “5% test.” The error is, indeed, negligible. ↵
- [PCl3] = 1.80 M; [PC3] = 0.195 M; [PCl3] = 0.195 M. ↵
- [NO2] = 0.19 M ; [NO] = 0.0070 M ; [O2] = 0.0035 M ↵
- [latex]P_{\text{O}_3} = 4.9\;\times\;10^{-26}\;\text{atm}[/latex] ↵
- 507 g ↵
- 330 g ↵
- (a) Both gases must increase in pressure. (b)[latex]P_{\text{N}_2\text{O}_4} = 8.0\;\text{atm and}\;P_{\text{NO}_2} = 1.0\;\text{atm}[/latex] ↵
- (a) 0.33 mol. (b) [CO]2 = 0.50 M Added H2 forms some water to compensate for the removal of water vapour and as a result of a shift to the left after H2 is added. ↵
- [latex]P_{\text{H}_2} = 8.64\;\times\;10^{-11}\;\text{atm}[/latex] [latex]P_{\text{O}_2} = 0.250\;\text{atm}[/latex] [latex]P_{\text{H}_2\text{O}} = 0.500\;\text{atm}[/latex] ↵
- (a) [latex]K_c = \frac{[\text{NH}_3]^4[\text{O}_2]^7}{[\text{NO}_2]^4[\text{H}_2\text{O}]^6}[/latex]. (b) [NH3] must increase for Qc to reach Kc. (c) That decrease in pressure would decrease [NO2]. (d) [latex]P_{\text{O}_2} = 49\;\text{torr}[/latex] ↵
- [fructose] = 0.15 M ↵
- [latex]P_{\text{N}_2\text{O}_3} = 1.90\;\text{atm and}\;P_{\text{NO}} = P_{\text{NO}_2} = 1.90\;\text{atm}[/latex] ↵
- (a) HB ionizes to a greater degree and has the larger Kc. (b) Kc(HA) = 5 × 10−4 Kc(HB) = 3 × 10−3 ↵
- (a) [latex]\begin{array}{lccc} \text{AgI}(s)\;{\rightleftharpoons}\; & \text{Ag}^{+}(aq) & + & \text{I}^{-}(aq) \\[0.5em] & x & & \rule[-0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}x \end{array}[/latex] (b) [latex]\begin{array}{lccc} \text{CaCO}_3(s)\;{\rightleftharpoons} & \text{Ca}^{2+}(aq) & + & \text{CO}_3^{\;\;2-}(aq) \\[0.5em] & \rule[-0.25ex]{0.5em}{0.1ex}\hspace{-0.5em}x & & x \end{array}[/latex] (c) [latex]\begin{array}{lccc} \text{Mg(OH)}_2(s)\;{\rightleftharpoons} & \text{Mg}^{2+}(aq) & + & 2\text{OH}^{-}(aq) \\[0.5em] & x & & \rule[-0.25ex]{1em}{0.1ex}\hspace{-1em}2x \end{array}[/latex] (d) [latex]\begin{array}{lccc} \text{Mg}_3(\text{PO}_4)_2(s)\;{\rightleftharpoons} & 3\text{Mg}^{2+}(aq) & + & 2\text{PO}_4^{\;\;3-}(aq) \\[0.5em] & \rule[-0.25ex]{1em}{0.1ex}\hspace{-1em}3x & & 2x \end{array}[/latex] (e) [latex]\begin{array}{lccccc} \text{Ca}_5(\text{PO}_4)_3\text{OH}(s)\;{\rightleftharpoons} & 5\text{Ca}^{2+}(aq) & + & 3\text{PO}_4^{\;\;3-}(aq) & + & \text{OH}^{-}(aq) \\[0.5em] & \rule[-0.25ex]{1em}{0.1ex}\hspace{-1em}5x & & \rule[-0.25ex]{1em}{0.1ex}\hspace{-1em}3x & & x \end{array}[/latex] ↵
- There is no change. A solid has an activity of 1 whether there is a little or a lot. ↵
- The solubility of silver bromide at the new temperature must be known. Normally the solubility increases and some of the solid silver bromide will dissolve. ↵
- CaF2, MnCO3, and ZnS ↵
- (a) [latex]\text{LaF}_3(s)\;{\rightleftharpoons}\;\text{La}^{3+}(aq)\;+\;3\text{F}^{-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = [\text{La}^{3+}][\text{F}^{-}]^3[/latex]; (b) [latex]\text{CaCO}_3(s)\;{\rightleftharpoons}\;\text{Ca}^{2+}(aq)\;+\;\text{CO}_3^{\;\;2-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = [\text{Ca}^{2+}][\text{CO}_3^{\;\;2-}][/latex]; (c) [latex]\text{Ag}_2\text{SO}_4(s)\;{\rightleftharpoons}\;2\text{Ag}^{+}(aq)\;+\;\text{SO}_4^{\;\;2-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = [\text{Ag}^{+}]^2[\text{SO}_4^{\;\;2-}][/latex]; (d) [latex]\text{Pb(OH)}_2(s)\;{\rightleftharpoons}\;\text{Pb}^{2+}(aq)\;+\;2\text{OH}^{-}(aq)\;\;\;\;\;\;\;K_{\text{sp}} = [\text{Pb}^{2+}][\text{OH}^{-}]^2[/latex] ↵
- (a)1.77 × 10–7; (b) 1.6 × 10–6; (c) 2.2 × 10–9; (d) 7.91 × 10–22 ↵
- (a) 2 × 10–2M; (b) 1.5 × 10–3M; (c) 2.27 × 10–9M; (d) 2.2 × 10–10M ↵
- (a) 6.4 × 10−9M = [Ag+], [Cl−] = 0.025 M Check: [latex]\frac{6.4\;\times\;10^{-9}\;M}{0.025\;M}\;\times\;100\% = 2.6\;\times\;10^{-5}\;%[/latex],an insignificant change; (b) 2.2 × 10−5M = [Ca2+], [F−] = 0.0013 M Check: [latex]\frac{2.26\;\times\;10^{-5}\;M}{0.00133\;M}\;\times\;100\% = 1.70\%[/latex]. This value is less than 5% and can be ignored. (c) 0.2238 M = [latex][\text{SO}_4^{\;\;2-}][/latex]; [Ag+] = 7.4 × 10–3M Check: [latex]\frac{3.7\;\times\;10^{-3}}{0.2238}\;\times\;100\% = 1.64\;\times\;10^{-2}\%[/latex]; the condition is satisfied. (d) [OH–] = 2.8 × 10–3M; 5.7 × 10−12M = [Zn2+] Check: [latex]\frac{5.7\;\times\;10^{-12}}{2.8\;\times\;10^{-3}}\;\times\;100\% = 2.0\;\times\;10^{-7}\%[/latex]; x is less than 5% of [OH–] and is, therefore, negligible. ↵
-
(a) [Cl–] = 7.6 × 10−3M Check: [latex]\frac{7.6\;\times\;10^{-3}}{0.025}\;\times\;100\% = 30\%[/latex] This value is too large to drop x. Therefore solve by using the quadratic equation: [Ti+] = 3.1 × 10–2M [Cl–] = 6.1 × 10–3
(b) [Ba2+] = 7.7 × 10–4M Check: [latex]\frac{7.7\;\times\;10^{-4}}{0.0313}\;\times\;100\% = 2.4\%[/latex] Therefore, the condition is satisfied. [Ba2+] = 7.7 × 10–4M [F–] = 0.0321 M; (c) Mg(NO3)2 = 0.02444 M [latex][\text{C}_2\text{O}_4^{\;\;2-}] = 2.9\;\times\;10^{-5}[/latex] Check: [latex]\frac{2.9\;\times\;10^{-5}}{0.02444}\;\times\;100\% = 0.12\%[/latex] The condition is satisfied; the above value is less than 5%. [latex][\text{C}_2\text{O}_4^{\;\;2-}] = 2.9\;\times\;10^{-5}\;M[/latex] [Mg2+] = 0.0244 M (d) [OH–] = 0.0501 M [Ca2+] = 3.15 × 10–3 Check: [latex]\frac{3.15\;\times\;10^{-3}}{0.050}\;\times\;100\% = 6.28\%[/latex] This value is greater than 5%, so a more exact method, such as successive approximations, must be used. [Ca2+] = 2.8 × 10–3M [OH–] = 0.053 × 10–2M ↵ - CaSO4∙2H2O is the most soluble Ca salt in mol/L, and it is also the most soluble Ca salt in g/L. ↵
- 4.8 × 10–3M = [latex][\text{SO}_4^{\;\;2-}][/latex] = [Ca2+]; Since this concentration is higher than 2.60 × 10–3M, “gyp” water does not meet the standards. ↵
- Mass (CaSO4·2H2O) = 0.72 g/L ↵
- (a) [Ag+] = [I–] = 1.3 × 10–5M; (b) [Ag+] = 2.88 × 10–2M, [latex][\text{SO}_4^{\;\;2-}][/latex] = 1.44 × 10–2M; (c) [Mn2+] = 3.7 × 10–5M, [OH–] = 7.4 × 10–5M; (d) [Sr2+] = 4.3 × 10–2M, [OH–] = 8.6 × 10–2M; (e) [Mg2+] = 1.3 × 10–4M, [OH–] = 2.6 × 10–4M. ↵
- (a) 2.0 × 10–4; (b) 5.1 × 10–17; (c) 1.35 × 10–4; (d) 1.18 × 10–5; (e) 1.08 × 10–10 ↵
- (a) CaCO3 does precipitate. (b) The compound does not precipitate. (c) The compound does not precipitate. (d) The compound precipitates. ↵
- 3.03 × 10−7M ↵
- 9.2 × 10−13M ↵
- [Ag+] = 1.8 × 10–3M ↵
- 6.3 × 10–4 ↵
- (a) 2.25 L; (b) 7.2 × 10–7 g ↵
- 100% of it is dissolved ↵
- (a) [latex]\text{Hg}_2^{\;\;2+}[/latex] and Cu2+: Add [latex]\text{SO}_4^{\;\;2-}[/latex]. (b) [latex]\text{SO}_4^{\;\;2-}[/latex] and Cl–: Add Ba2+. (c) Hg2+ and Co2+: Add S2–. (d) Zn2+ and Sr2+: Add OH– until [OH–] = 0.050 M. (e) Ba2+ and Mg2+: Add [latex]\text{SO}_4^{\;\;2-}[/latex]. (f) [latex]\text{CO}_3^{\;\;2-}[/latex] and OH–: Add Ba2+. ↵
- AgI will precipitate first. ↵
- 1.5 × 10−12M ↵
- 3.99 kg ↵
- (a) 3.1 × 10–11; (b) [Cu2+] = 2.6 × 10–3; [latex][\text{IO}_3^{\;\;-}][/latex] = 5.3 × 10–3 ↵
- 1.8 × 10–5 g Pb(OH)2 ↵
-
[latex]\text{Mg(OH)}_2(s)\;{\rightleftharpoons}\;\text{Mg}^{2+}\;+\;2\text{OH}^{-}\;\;\;\;\;\;\;K_{\text{sp}} = [\text{Mg}^{2+}][\text{OH}^{-}]^2[/latex]
1.23 × 10−3 g Mg(OH)2 ↵ - MnCO3 will form first, since it has the smallest Ksp value it is the least soluble. MnCO3 will be the last to precipitate, it has the largest Ksp value. ↵
- The salt ionizes in solution, but the anion slightly reacts with water to form the weak acid. This reaction also forms OH−, which causes the solution to be basic. ↵
- [latex][\text{H}_2\text{O}]\;\text{>}\;[\text{CH}_3\text{CO}_2\text{H}]\;\text{>}\;[\text{H}_3\text{O}^{+}]\;{\approx}\;[\text{CH}_3\text{CO}_2^{\;\;-}]\;\text{>}\;[\text{OH}^{-}][/latex] ↵
- The oxidation state of the sulfur in H2SO4 is greater than the oxidation state of the sulfur in H2SO3. ↵
- [latex]\begin{array}{ccccccccc} \text{Mg(OH)}_2(s) & + & 2\text{HCl}(aq) & {\longrightarrow} & \text{Mg}^{2+}(aq) & + & 2\text{Cl}^{-}(aq) & + & 2\text{H}_2\text{O}(l) \\[0.5em] \text{BB} & & \text{BA} & & \text{CB} & & \text{CA} & & \end{array}[/latex]holy ↵
- [latex]K_{\text{a}} = 2.3\;\times\;10^{-11}[/latex] ↵
- The stronger base or stronger acid is the one with the larger Kb or Ka, respectively. In these two examples, they are (CH3)2NH and [latex]\text{CH}_3\text{NH}_3^{\;\;+}[/latex]. ↵
- triethylamine. ↵
- (a) [latex]\text{HSO}_4^{\;\;-}[/latex]; higher electronegativity of the central ion. (b) H2O; NH3 is a base and water is neutral, or decide on the basis of Ka values. (c) HI; PH3 is weaker than HCl; HCl is weaker than HI. Thus, PH3 is weaker than HI. (d) PH3; in binary compounds of hydrogen with nonmetals, the acidity increases for the element lower in a group. (e) HBr; in a period, the acidity increases from left to right; in a group, it increases from top to bottom. Br is to the left and below S, so HBr is the stronger acid. ↵
- (a) [latex]\text{NaHSeO}_3\;\text{<}\;\text{NaHSO}_3\;\text{<}\;\text{NaHSO}_4[/latex]; in polyoxy acids, the more electronegative central element—S, in this case—forms the stronger acid. The larger number of oxygen atoms on the central atom (giving it a higher oxidation state) also creates a greater release of hydrogen atoms, resulting in a stronger acid. As a salt, the acidity increases in the same manner. (b) [latex]\text{ClO}_2^{\;\;-}\;\text{<}\;\text{BrO}_2^{\;\;-}\;\text{<}\;\text{IO}_2^{\;\;-}[/latex]; the basicity of the anions in a series of acids will be the opposite of the acidity in their oxyacids. The acidity increases as the electronegativity of the central atom increases. Cl is more electronegative than Br, and I is the least electronegative of the three. (c) [latex]\text{HOI}\;\text{<}\;\text{HOBr}\;\text{<}\;\text{HOCl}[/latex]; in a series of the same form of oxyacids, the acidity increases as the electronegativity of the central atom increases. Cl is more electronegative than Br, and I is the least electronegative of the three. (d) [latex]\text{HOCl}\;\text{<}\;\text{HOClO}\;\text{<}\;\text{HOClO}_2\;\text{<}\;\text{HOClO}_3[/latex]; in a series of oxyacids of the same central element, the acidity increases as the number of oxygen atoms increases (or as the oxidation state of the central atom increases). (e) [latex]\text{HTe}^{-}\;\text{<}\;\text{HS}^{-}\;{\ll}\;\text{PH}_2^{\;\;-}\;\text{<}\;\text{NH}_2^{\;\;-}[/latex]; [latex]\text{PH}_2^{\;\;-}[/latex] and [latex]\text{NH}_2^{\;\;-}[/latex] are anions of weak bases, so they act as strong bases toward H<sup>+. HTe− and HS− are anions of weak acids, so they have less basic character. In a periodic group, the more electronegative element has the more basic anion. (f) [latex]\text{BrO}_4^{\;\;-}\;\text{<}\;\text{BrO}_3^{\;\;-}\;\text{<}\;\text{BrO}_2^{\;\;-}\;\text{<}\;\text{BrO}^{-}[/latex]; with a larger number of oxygen atoms (that is, as the oxidation state of the central ion increases), the corresponding acid becomes more acidic and the anion consequently less basic. ↵
- [latex][\text{H}_2\text{O}]\;\text{>}\;[\text{C}_6\text{H}_4\text{OH}(\text{CO}_2\text{H})]\;\text{>}\;[\text{H}^{+}]_{0}\;\text{>}\;[\text{C}_6\text{H}_4\text{OH}(\text{CO}_2)^{-}]\;{\gg}\;[\text{C}_6\text{H}_4\text{O}(\text{CO}_2\text{H})^{-}]\;\text{>}\;[\text{OH}^{-}][/latex] ↵
- Strong electrolytes are 100% ionized, and, as long as the component ions are neither weak acids nor weak bases, the ionic species present result from the dissociation of the strong electrolyte. Equilibrium calculations are necessary when one (or more) of the ions is a weak acid or a weak base. ↵
- 1. Assume that the change in initial concentration of the acid as the equilibrium is established can be neglected, so this concentration can be assumed constant and equal to the initial value of the total acid concentration. 2. Assume we can neglect the contribution of water to the equilibrium concentration of H3O+. ↵
- (b) The addition of HCl ↵
- (a) Adding HCl will add H3O+ ions, which will then react with the OH− ions, lowering their concentration. The equilibrium will shift to the right, increasing the concentration of HNO2, and decreasing the concentration of [latex]\text{NO}_2^{\;\;-}[/latex] ions. (b) Adding HNO2 increases the concentration of HNO2 and shifts the equilibrium to the left, increasing the concentration of [latex]\text{NO}_2^{\;\;-}[/latex] ions and decreasing the concentration of OH− ions. (c) Adding NaOH adds OH− ions, which shifts the equilibrium to the left, increasing the concentration of [latex]\text{NO}_2^{\;\;-}[/latex] ions and decreasing the concentrations of HNO2. (d) Adding NaCl has no effect on the concentrations of the ions. (e) Adding KNO2 adds [latex]\text{NO}_2^{\;\;-}[/latex] ions and shifts the equilibrium to the right, increasing the HNO2 and OH− ion concentrations. ↵
- This is a case in which the solution contains a mixture of acids of different ionization strengths. In solution, the HCO2H exists primarily as HCO2H molecules because the ionization of the weak acid is suppressed by the strong acid. Therefore, the HCO2H contributes a negligible amount of hydronium ions to the solution. The stronger acid, HCl, is the dominant producer of hydronium ions because it is completely ionized. In such a solution, the stronger acid determines the concentration of hydronium ions, and the ionization of the weaker acid is fixed by the [H3O+] produced by the stronger acid. ↵
- (a) [latex]K_{\text{b}} = 1.8\;\times\;10^{-5}[/latex]; (b) [latex]K_{\text{a}} = 4.5\;\times\;10^{-4}[/latex]; (c) [latex]K_{\text{b}} = 7.4\;\times\;10^{-5}[/latex]; (d) [latex]K_{\text{a}} = 5.6\;\times\;10^{-10}[/latex] ↵
- [latex]K_{\text{a}} = 1.2\;\times\;10^{-2}[/latex] ↵
- (a) [latex]K_{\text{b}} = 4.3\;\times\;10^{-12}[/latex]; (b) [latex]K_{\text{a}} = 1.6\;\times\;10^{-8}[/latex]; (c) [latex]K_{\text{b}} = 5.9\;\times\;10^{-7}[/latex]; (d) [latex]K_{\text{b}} = 4.2\;\times\;10^{-3}[/latex]; (e) [latex]K_{\text{b}} = 2.3\;\times\;10^{-3}[/latex]; (f) [latex]K_{\text{b}} = 6.3\;\times\;10^{-13}[/latex] ↵
- (a) is the correct statement. ↵
- [H3O+] = 7.5 × 10−3M [HNO2] = 0.127 [OH−] = 1.3 × 10−12M [BrO−] = 4.5 × 10−8M [HBrO] = 0.120 M ↵
- [OH−] = [latex][\text{NO}_4^{\;\;+}][/latex] = 0.0014 M [NH3] = 0.144 M [H3O+] = 6.9 × 10−12M [latex][\text{C}_6\text{H}_5\text{NH}_3^{\;\;+}][/latex] = 3.9 × 10−8M [C6H5NH2] = 0.100 M ↵
- (a) [latex]\frac{[\text{H}_3\text{O}^{+}][\text{ClO}^{-}]}{[\text{HClO}]} = \frac{(x)(x)}{(0.0092\;-\;x)}\;{\approx}\;\frac{(x)(x)}{0.0092} = 2.9\;\times\;10^{-8}[/latex] Solving for x gives 1.63 × 10−5M. This value is less than 5% of 0.0092, so the assumption that it can be neglected is valid. Thus, the concentrations of solute species at equilibrium are: [H3O+] = [ClO] = 5.8 × 10−5M [HClO] = 0.00092 M [OH−] = 6.1 × 10−10M; (b) [latex]\frac{[\text{C}_6\text{H}_5\text{NH}_3^{\;\;+}][\text{OH}^{-}]}{[\text{C}_6\text{H}_5\text{NH}_2]} = \frac{(x)(x)}{(0.0784\;-\;x)}\;{\approx}\;\frac{(x)(x)}{0.0784} = 4.3\;\times\;10^{-10}[/latex] Solving for x gives 5.81 × 10−6M. This value is less than 5% of 0.0784, so the assumption that it can be neglected is valid. Thus, the concentrations of solute species at equilibrium are: [latex][\text{CH}_3\text{CO}_2^{\;\;-}][/latex] = [OH−] = 5.8 × 10−6M [C6H5NH2] = 0.00784 [H3O+] = 1.7× 10−9M;(c) [latex]\frac{[\text{H}_3\text{O}^{+}][\text{CN}^{-}]}{[\text{HCN}]} = \frac{(x)(x)}{(0.0810\;-\;x)}\;{\approx}\;\frac{(x)(x)}{0.0810} = 4.9\;\times\;10^{-10}[/latex] Solving for x gives 6.30 × 10−6M. This value is less than 5% of 0.0810, so the assumption that it can be neglected is valid. Thus, the concentrations of solute species at equilibrium are: [H3O+] = [CN−] = 6.3 × 10−6M [HCN] = 0.0810 M [OH−] = 1.6 × 10−9M;(d) [latex]\frac{[(\text{CH}_3)_3\text{NH}^{+}][\text{OH}^{-}]}{[(\text{CH}_3)_3\text{N}]} = \frac{(x)(x)}{(0.11\;-\;x)}\;{\approx}\;\frac{(x)(x)}{0.11} = 6.3\;\times\;10^{-5}[/latex] Solving for x gives 2.63 × 10−3M. This value is less than 5% of 0.11, so the assumption that it can be neglected is valid. Thus, the concentrations of solute species at equilibrium are: [(CH3)3NH+] = [OH−] = 2.6 × 10−3M [(CH3)3N] = 0.11 M [H3O+] = 3.8 × 10−12M;(e) [latex]\frac{[\text{Fe}(\text{H}_2\text{O})_5(\text{OH})^{+}][\text{H}_3\text{O}^{+}]}{[\text{Fe}(\text{H}_2\text{O})_6^{\;\;2+}]} = \frac{(x)(x)}{(0.120\;-\;x)}\;{\approx}\;\frac{(x)(x)}{0.120} = 1.6\;\times\;10^{-7}[/latex] Solving for x gives 1.39 × 10−4M. This value is less than 5% of 0.120, so the assumption that it can be neglected is valid. Thus, the concentrations of solute species at equilibrium are: [Fe(H2O)5(OH)+] = [H3O+] = 1.4 × 10−4M [latex][\text{Fe}(\text{H}_2\text{O})_6^{\;\;2+}][/latex] = 0.120 M [OH−] = 7.2 × 10−11M ↵
- pH = 2.41 ↵
- [C10H14N2] = 0.049 M [C10H14N2H+] = 1.9 × 10−4M [latex][\text{C}_{10}\text{H}_{14}\text{N}_2\text{H}_2^{\;\;2+}][/latex] = 1.4 × 10−11M [OH−] = 1.9 × 10−4M [H3O+] = 5.3 × 10−11M ↵
- [latex]K_{\text{a}} = 1.2\;\times\;10^{-2}[/latex] ↵
- [latex]K_{\text{b}} = 1.77\;\times\;10^{-5}[/latex] ↵
- 0.014 M ↵
- 7.2 × 10–15M ↵
- 4.4 × 10−22M ↵
- 6.2 × 10–6M = [Hg2+]; 1.2 × 10–5M = [Cl–]; The substance is a weak electrolyte because very little of the initial 0.015 M HgCl2 dissolved. ↵
- [OH−] = 4.5 × 10−5; [Al3+] = 2.2 × 10–20 (molar solubility) ↵
- [latex][\text{SO}_4^{\;\;2-}] = 0.049\;M[/latex] [Ba2+] = 4.7 × 10–7 (molar solubility) ↵
- [OH–] = 7.6 × 10−3M [Pb2+] = 2.1 × 10–11 (molar solubility) ↵
- 7.66 ↵
- [latex][\text{CO}_3^{\;\;2-}] = 0.116\;M[/latex] [Cd2+] = 4.5 × 10−11M ↵
- 3.1 × 10−3M ↵
- 0.0102 L (10.2 mL) ↵
- 5.4 × 10−3 g ↵
- (a) Ksp = [Mg2+][F–]2 = (1.21 × 10–3)(2 × 1.21 × 10–3)2 = 7.09 × 10–9; (b) 7.09 × 10–7M (c) Determine the concentration of Mg2+ and F– that will be present in the final volume. Compare the value of the ion product [Mg2+][F–]2 with Ksp. If this value is larger than Ksp, precipitation will occur. 0.1000 L × 3.00 × 10–3M Mg(NO3)2 = 0.3000 L ×M Mg(NO3)2M Mg(NO3)2 = 1.00 × 10–3M 0.2000 L × 2.00 × 10–3M NaF = 0.3000 L ×M NaF M NaF = 1.33 × 10–3M ion product = (1.00 × 10–3)(1.33 × 10–3)2 = 1.77 × 10–9 This value is smaller than Ksp, so no precipitation will occur. (d) MgF2 is less soluble at 27 °C than at 18 °C. Because added heat acts like an added reagent, when it appears on the product side, the Le Châtelier’s principle states that the equilibrium will shift to the reactants’ side to counter the stress. Consequently, less reagent will dissolve. This situation is found in our case. Therefore, the reaction is exothermic. ↵
- BaF2, Ca3(PO4)2, ZnS; each is a salt of a weak acid, and the [latex][\text{H}_3\text{O}^{+}][/latex] from perchloric acid reduces the equilibrium concentration of the anion, thereby increasing the concentration of the cations ↵
- Effect on amount of solid CaHPO4, [Ca2+], [OH–]: (a) increase, increase, decrease; (b) decrease, increase, decrease; (c) no effect, no effect, no effect; (d) decrease, increase, decrease; (e) increase, no effect, no effect ↵
- 9.2 g ↵

