4.1 Physical and Chemical Properties
Learning Objectives
By the end of this section, you will be able to:
- Identify properties of and changes in matter as physical or chemical
- Distinguish between physical and chemical properties
- Identify properties of matter as extensive or intensive
The characteristics that enable us to distinguish one substance from another are called properties. A physical property is a characteristic of matter that is not associated with a change in its chemical composition. Familiar examples of physical properties include density, colour, hardness, melting and boiling points, and electrical conductivity. We can observe some physical properties, such as density and colour, without changing the physical state of the matter observed. Other physical properties, such as the melting temperature of iron or the freezing temperature of water, can only be observed as matter undergoes a physical change.
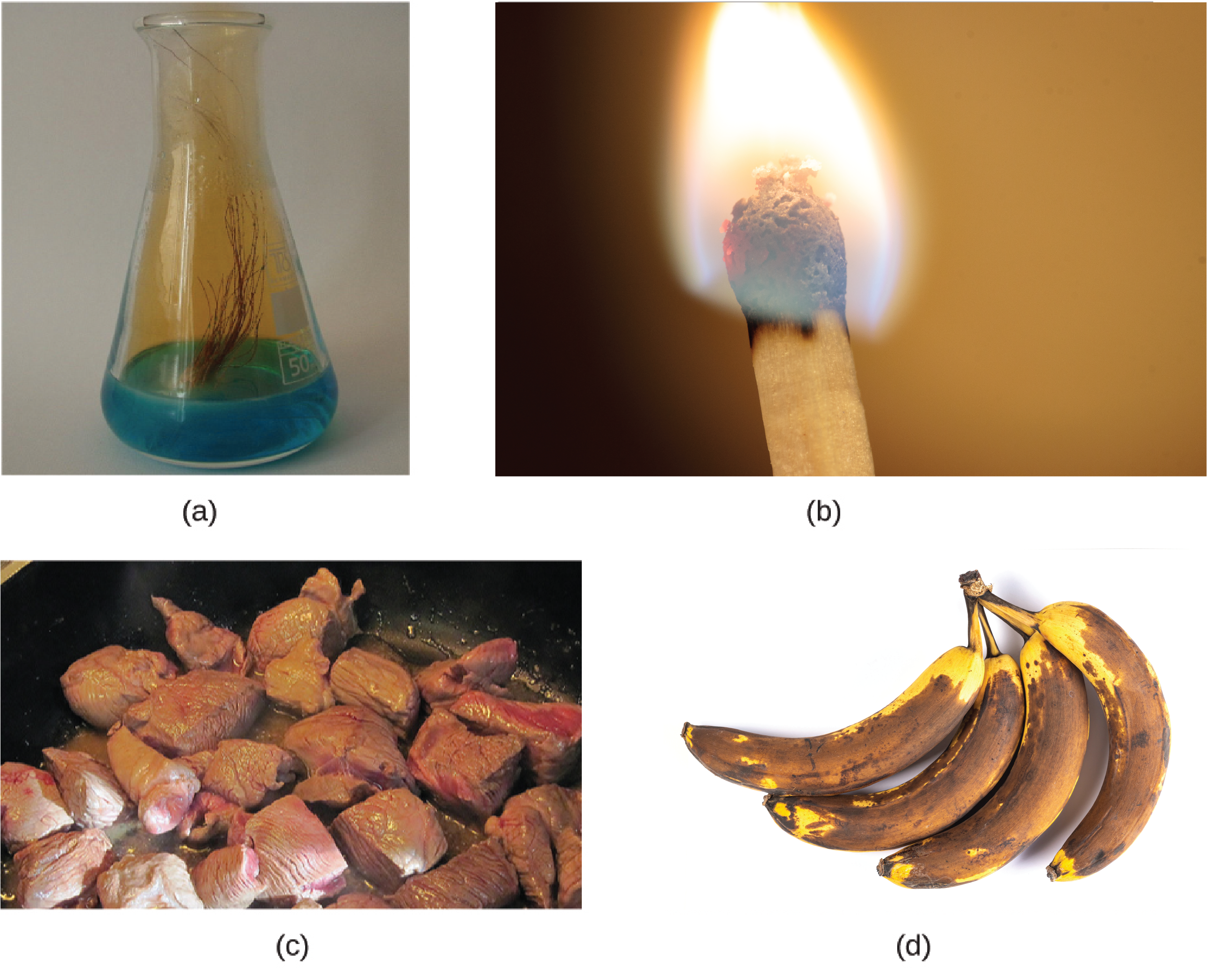
A physical change is a change in the state or properties of matter without any accompanying change in its chemical composition (the identities of the substances contained in the matter). We observe a physical change when wax melts, when sugar dissolves in coffee, and when steam condenses into liquid water (Figure 4.1a). Other examples of physical changes include magnetizing and demagnetizing metals (as is done with common antitheft security tags) and grinding solids into powders (which can sometimes yield noticeable changes in colour). In each of these examples, there is a change in the physical state, form, or properties of the substance, but no change in its chemical composition.

The change of one type of matter into another type (or the inability to change) is a chemical property. Examples of chemical properties include flammability, toxicity, acidity, reactivity (many types), and heat of combustion. Iron, for example, combines with oxygen in the presence of water to form rust; chromium does not oxidize (Figure 4.1b). Nitroglycerin is very dangerous because it explodes easily; neon poses almost no hazard because it is very unreactive.

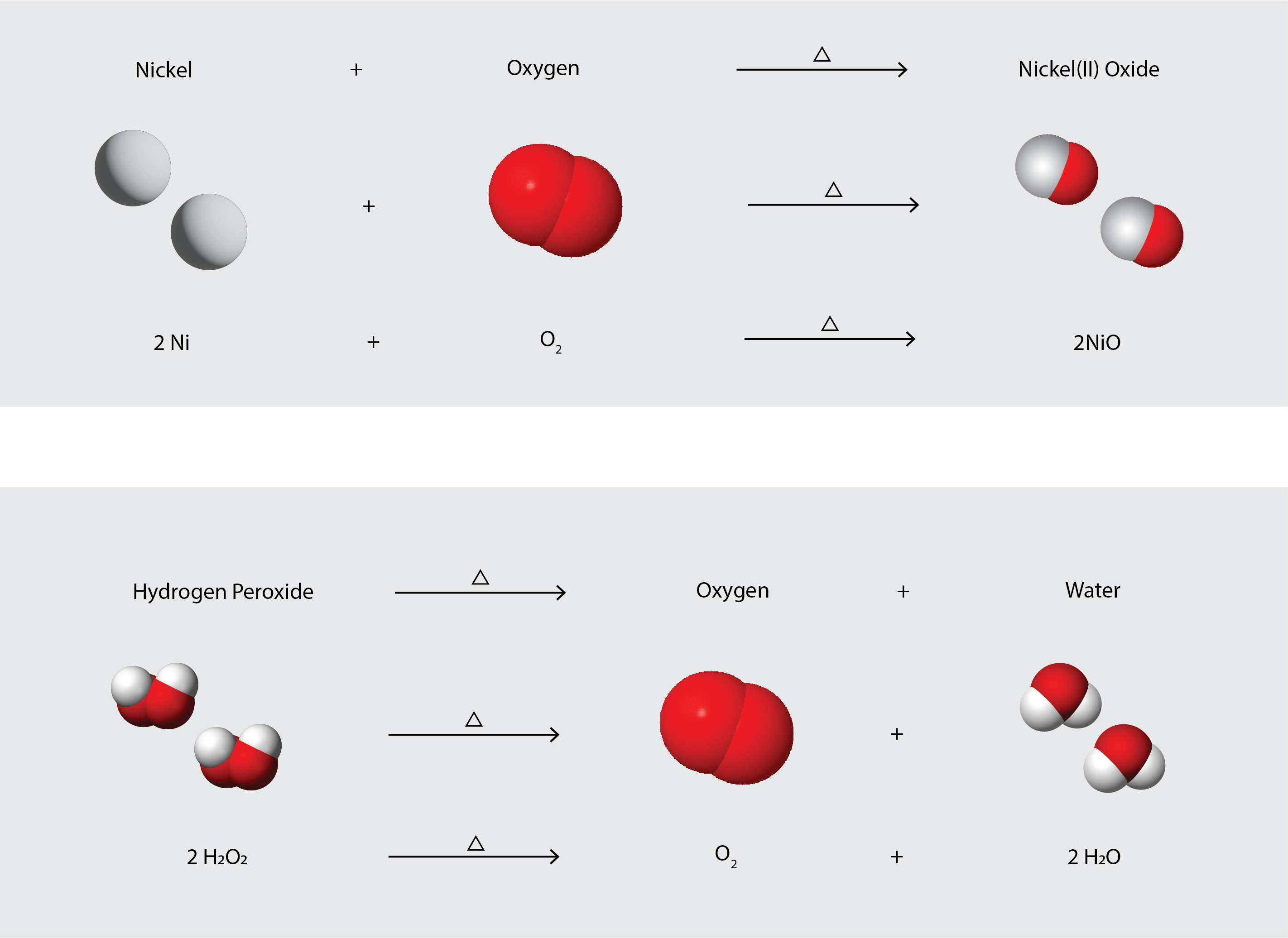
To identify a chemical property, we look for a chemical change. A chemical change always produces one or more types of matter that differ from the matter present before the change. The formation of rust is a chemical change because rust is a different kind of matter than the iron, oxygen, and water present before the rust formed. The explosion of nitroglycerin is a chemical change because the gases produced are very different kinds of matter from the original substance. Other examples of chemical changes include reactions that are performed in a lab (such as copper reacting with nitric acid), all forms of combustion (burning), and food being cooked, digested, or rotting (Figure 4.1c). Figure 4.1d illustrates two different chemical changes occurring, and represents each of them in several different ways: in words, as a space-filling diagram (which shows relative sizes of atoms), and as a chemical equation, which is a shorthand method for describing chemical changes.
Properties of matter fall into one of two categories. If the property depends on the amount of matter present, it is an extensive property. The mass and volume of a substance are examples of extensive properties; for instance, a gallon of milk has a larger mass and volume than a cup of milk. The value of an extensive property is directly proportional to the amount of matter in question. If the property of a sample of matter does not depend on the amount of matter present, it is an intensive property. Temperature is an example of an intensive property. If a gallon and cup of milk are each at 20 °C (room temperature), when they are combined the temperature remains at 20 °C. As another example, consider the distinct but related properties of heat and temperature. A drop of hot cooking oil spattered on your arm causes brief, minor discomfort, whereas a pot of hot oil yields severe burns. Both the drop and the pot of oil are at the same temperature (an intensive property), but the pot clearly contains much more heat (extensive property).
Hazard Diamond
You may have seen the symbol shown in Figure 4.1e on containers of chemicals in a laboratory or workplace. Sometimes called a “fire diamond” or “hazard diamond,” this chemical hazard diamond provides valuable information that briefly summarizes the various dangers of which to be aware when working with a particular substance.
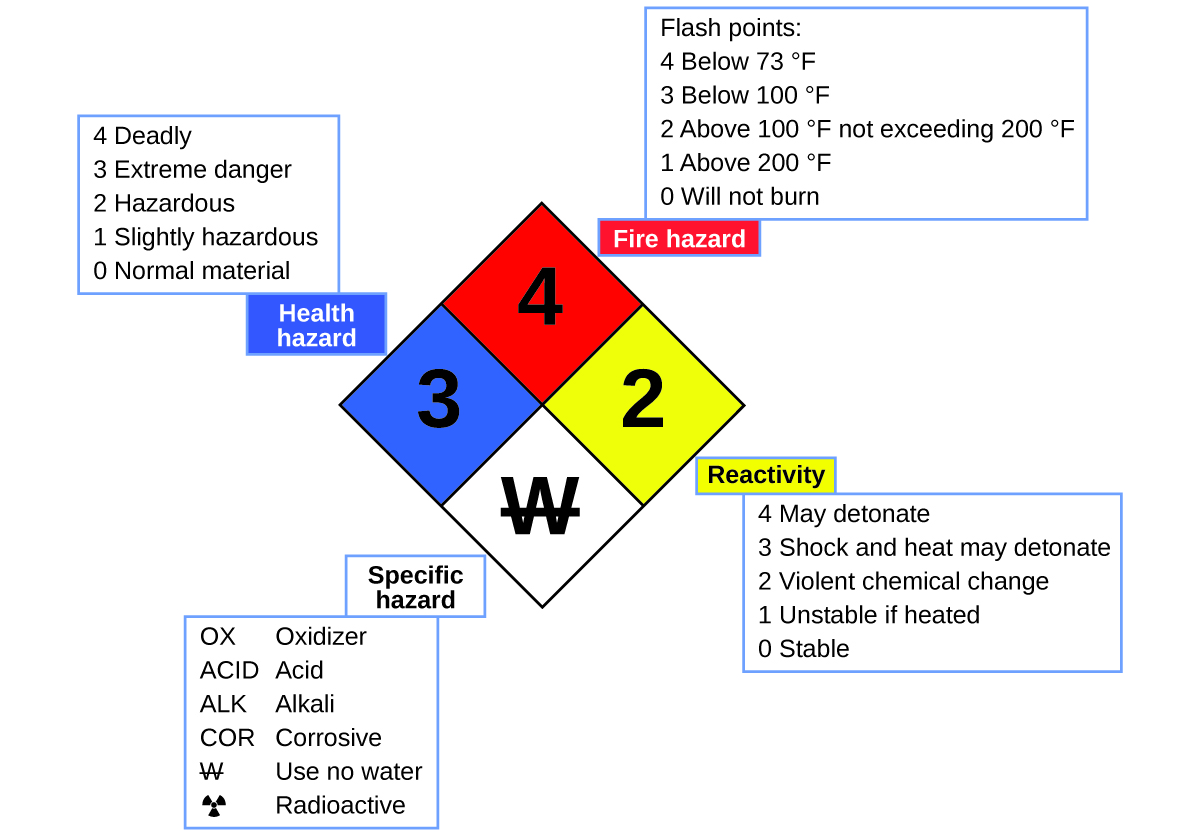
The National Fire Protection Agency (NFPA) 704 Hazard Identification System was developed by NFPA to provide safety information about certain substances. The system details flammability, reactivity, health, and other hazards. Within the overall diamond symbol, the top (red) diamond specifies the level of fire hazard (temperature range for flash point). The blue (left) diamond indicates the level of health hazard. The yellow (right) diamond describes reactivity hazards, such as how readily the substance will undergo detonation or a violent chemical change. The white (bottom) diamond points out special hazards, such as if it is an oxidizer (which allows the substance to burn in the absence of air/oxygen), undergoes an unusual or dangerous reaction with water, is corrosive, acidic, alkaline, a biological hazard, radioactive, and so on. Each hazard is rated on a scale from 0 to 4, with 0 being no hazard and 4 being extremely hazardous.
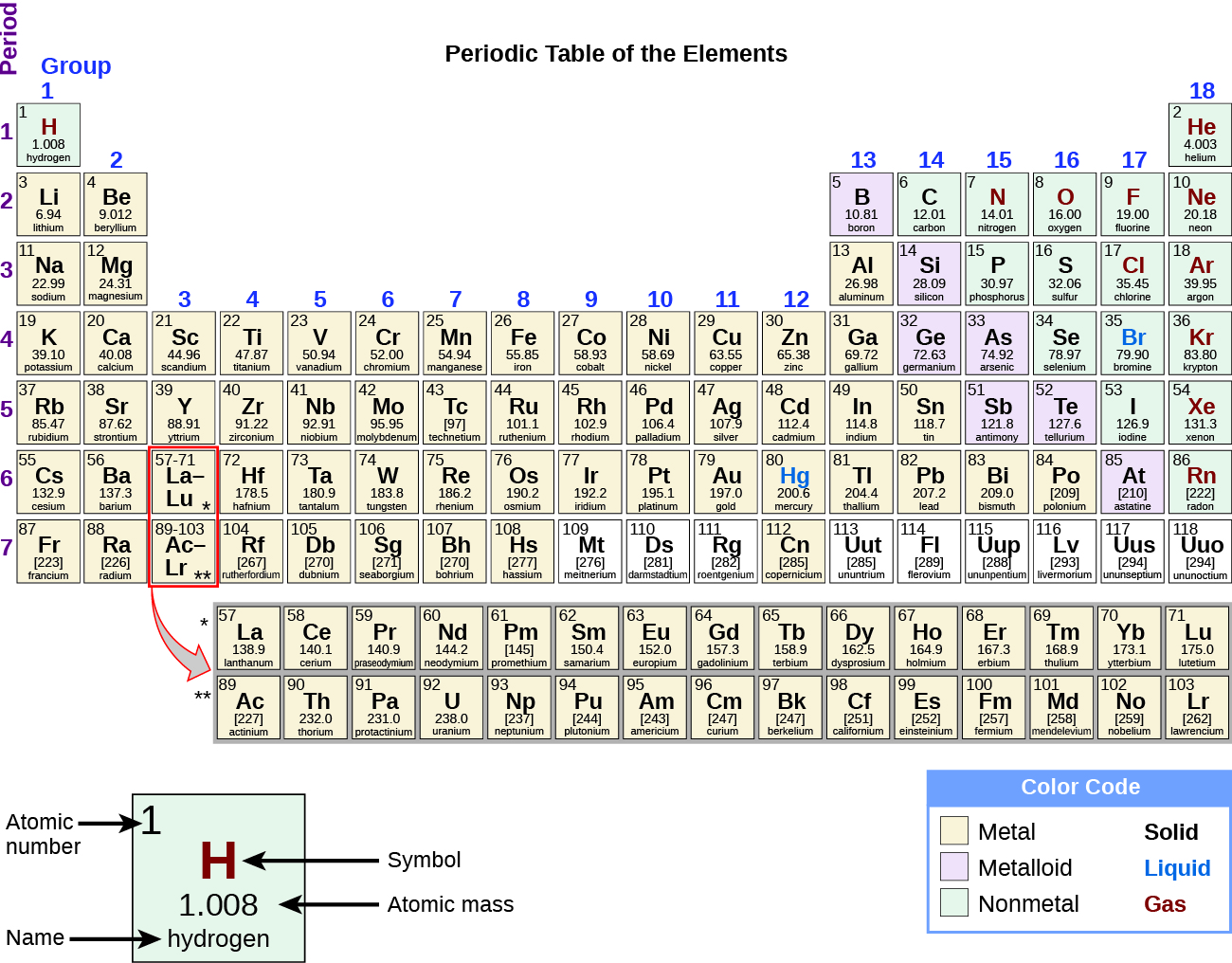
While many elements differ dramatically in their chemical and physical properties, some elements have similar properties. We can identify sets of elements that exhibit common behaviours. For example, many elements conduct heat and electricity well, whereas others are poor conductors. These properties can be used to sort the elements into three classes: metals (elements that conduct well), nonmetals (elements that conduct poorly), and metalloids (elements that have properties of both metals and nonmetals).
The periodic table is a table of elements that places elements with similar properties close together (Figure 4.1f). You will learn more about the periodic table as you continue your study of chemistry.
Exercise 4.1a: Drag the boxes to the appropriate heading
Check Your Learning Exercise (Text Version)
Classify each of the following descriptions as either a physical or chemical property.
- acid is corrosive to the skin
- the ice will melt at 10 degrees Celsius
- the sunburn is red
- hydrochloric acid is very acidic
- a person’s hair is brown
- arsenic is very poisonous
- insecticides kill insects
- water boils at 100 degrees Celsius
- the ice is cold
- the glass is not flammable
- helium gas is nonreactive
- the cake is very dense
Check Your Answer[1]
Source: “Exercise 4.1a” created by Jackie MacDonald, CC BY-NC-SA-4.0.
Exercise 4.1b
Check Your Learning Exercise (Text Version)
Classify each of the following as either a physical or chemical change:
- Condensation of steam
- Burning of gasoline
- Souring of milk
- Melting of gold
Check Your Answer[2]
Source: “Exercise 4.1b” is adapted from “Exercise 1.3-2” from General Chemistry 1 & 2, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson, licensed under CC BY 4.0.
Links to Interactive Learning Tools
Explore Chemical Vs. Physical Properties from the Physics Classroom.
Attribution & References
characteristic of matter that is not associated with any change in its chemical composition
change in the state or properties of matter that does not involve a change in its chemical composition
behavior that is related to the change of one kind of matter into another kind of matter
change producing a different kind of matter from the original kind of matter
property of a substance that depends on the amount of the substance
property of a substance that is independent of the amount of the substance

