Learning Objective
By the end of this section, you will be able to:
- To describe the unique characteristics of water in terms of its structure
Indigenous Perspective: Sea Ice and Snow

Inuit survival has always depended upon the important and stable coverage of the Arctic Ocean in ice. Sea ice (Figure 13.5a) is an essential gateway to freedom for the Inuit, and it makes life possible due to its many unique chemical properties. On the other hand, the chemistry of snow is equally important, especially given the fact that it covers the Arctic for most of the year. The chemical properties of snow make it an important insulator, building material, and an important cultural symbol for the Inuit.
Water: A Special Liquid
Earth is the only known body in our solar system that has liquid water existing freely on its surface. That is a good thing because life on Earth would not be possible without the presence of liquid water.
Water has several properties that make it a unique substance among substances. It is an excellent solvent; it dissolves many other substances and allows those substances to react when in solution. In fact, water is sometimes called the universal solvent because of this ability. Water has unusually high melting and boiling points (0°C and 100°C, respectively) for such a small molecule. The boiling points for similar-sized molecules, such as methane (BP = −162°C) and ammonia (BP = −33°C), are more than 100° lower. Though a liquid at normal temperatures, water molecules experience a relatively strong intermolecular interaction that allows them to maintain the liquid phase at higher temperatures than expected.
Unlike most substances, the solid form of water is less dense than its liquid form, which allows ice to float on water. The most energetically favourable configuration of H2O molecules is one in which each molecule is hydrogen-bonded to four neighbouring molecules. Owing to the thermal motions, this ideal is never achieved in the liquid, but when water freezes to ice, the molecules settle into exactly this kind of arrangement in the ice crystal. This arrangement requires that the molecules are somewhat farther apart than would otherwise be the case; as a consequence, ice, in which hydrogen bonding is at its maximum, has a more open structure, and thus a lower density than water (Figure 13.5b).
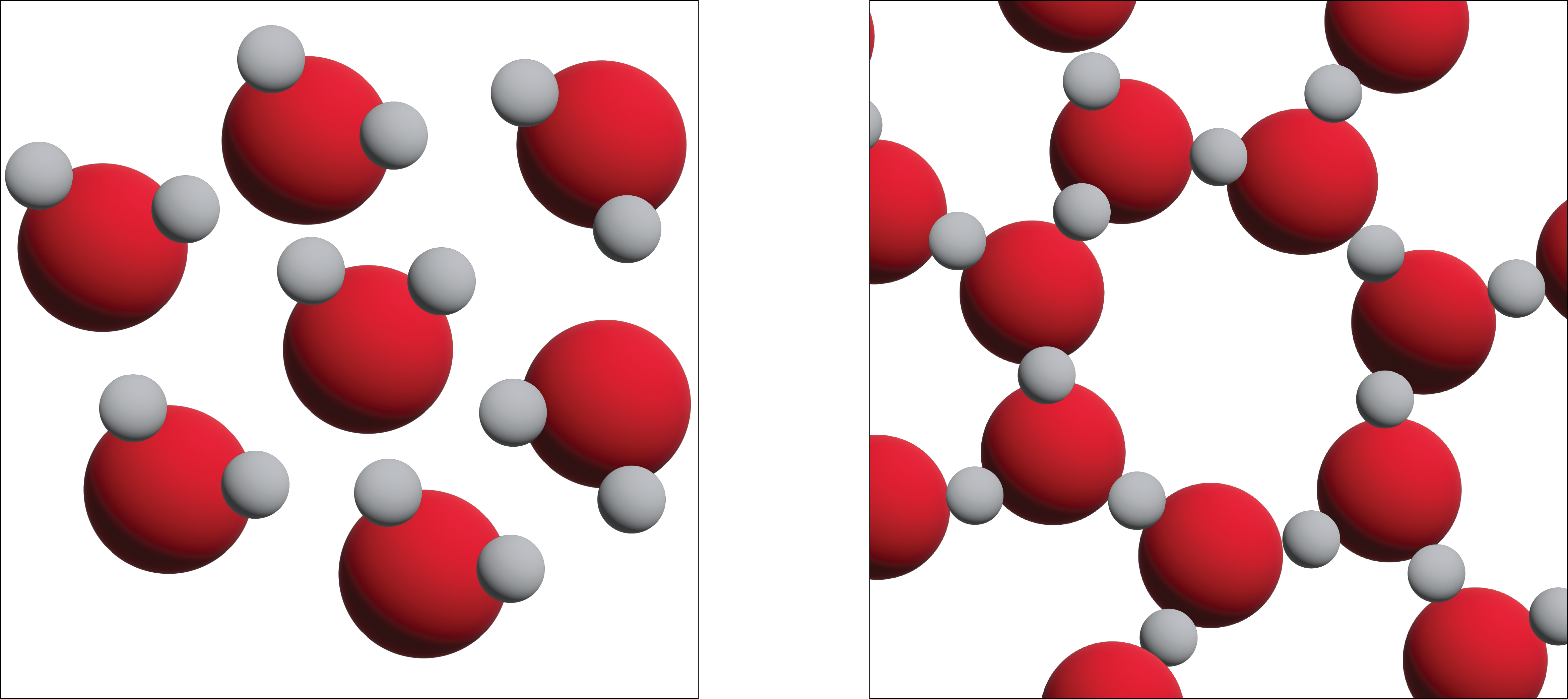
In Figure 13.5b we can see three-dimensional views of a typical local structure of water (left) and ice (right.) Notice the greater openness of the ice structure which is necessary to ensure the strongest degree of hydrogen bonding in a uniform, extended crystal lattice. The structure of liquid water is very similar, but in the liquid, the hydrogen bonds are continually broken and formed because of rapid molecular motion. Because ice is less dense than liquid water, rivers, lakes, and oceans freeze from the top down. In fact, the ice forms a protective surface layer that insulates the rest of the water, allowing fish and other organisms to survive in the lower levels of a frozen lake or sea. If ice were denser than the liquid, the ice formed at the surface in cold weather would sink as fast as it formed. Bodies of water would freeze from the bottom up, which would be lethal for most aquatic creatures. The expansion of water when freezing also explains why automobile or boat engines must be protected by “antifreeze” and why unprotected pipes in houses break if they are allowed to freeze.
Watch Why does ice float in water? (3:55 min)
Video source: TED-Ed. (2013, October 22). Why does ice float in water? – George Zaidan and Charles Morton [Video]. YouTube.
Water also requires an unusually large amount of energy to change temperature. While 100 J of energy will change the temperature of 1 g of iron by 230°C, this same amount of energy will change the temperature of 1 g of water by only 100°C. Thus, water changes its temperature slowly as heat is added or removed. This has a major impact on weather, as storm systems like hurricanes can be impacted by the amount of heat that ocean water can store. For more properties of water, refer to Appendix F.
Watch The Properties of Water (4:58 min)
Video source: Odyssey Earth. (2012, March 3). The Properties of Water [Video]. YouTube.
Structure of the Water Molecule
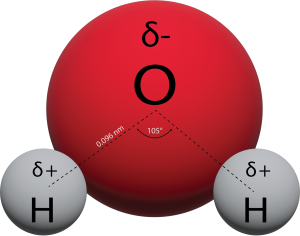
Water’s influence on the world around us is affected by these properties. Isn’t it fascinating that such a small molecule can have such a big impact?
Scientists in Action: Dr. Katsuko Saruhashi
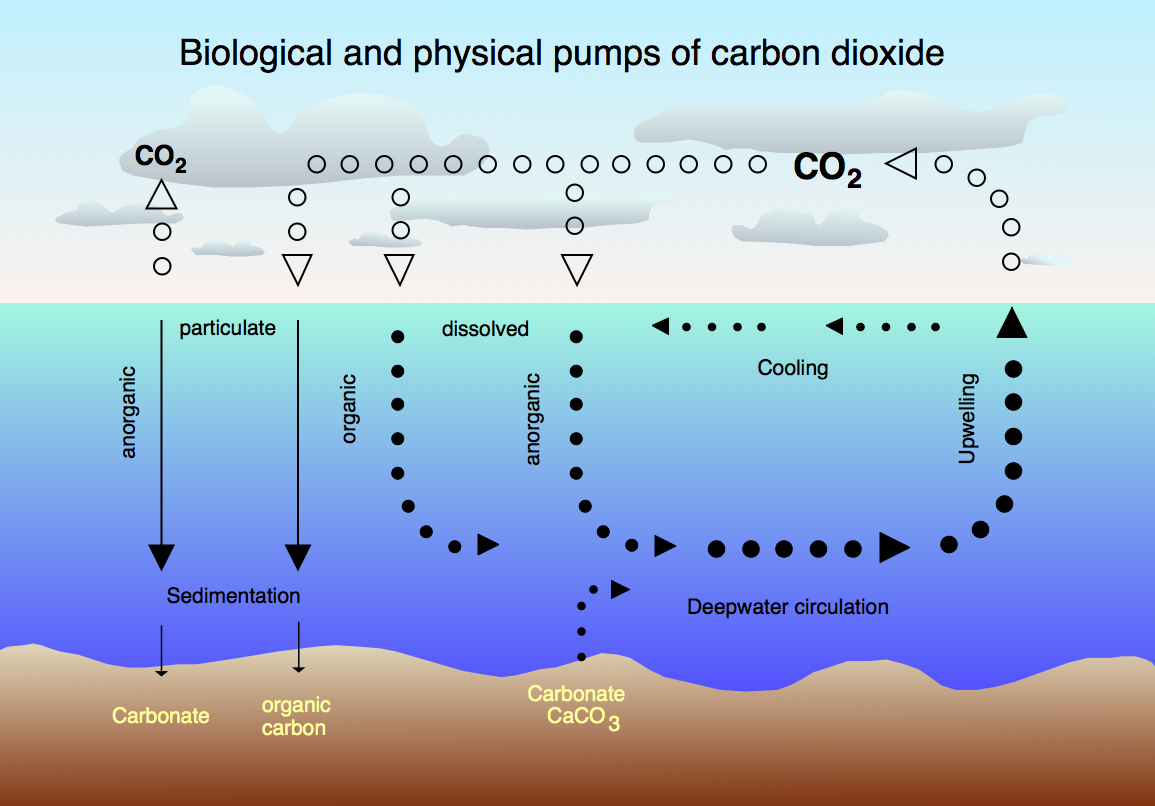
Japanese geochemist Katsuko Saruhashi (March 1920 – September 2007) was the first woman to earn a doctorate degree in chemistry from the prestigious University of Tokyo. Interested in environmental toxicology from an early age, Katsuko conducted research and created tools that let her take the first measurements of carbon dioxide (CO2) levels in seawater (Figure 13.5c). This groundbreaking research allowed her and her team to demonstrate that previous hypotheses based on the idea that elevated CO2 levels found in seawater could not be blamed on the dissolution of calcium carbonate, and instead were related to global warming caused by human activity. Among her many honours, she was the first woman elected to the Science Council of Japan and the first woman to be awarded the Miyake prize for geochemistry.
Read more about Katsuko Saruhashi in the article “Meet Katsuko Saruhashi, a resilient geochemist who detected nuclear fallout in the Pacific” [New Tab]
Watch The science of snowflakes (4:29 min)
Attribution & References
Except where otherwise noted, this section is adapted by Gregory A. Anderson from “8.2: Solids and Liquids” In Basics of General, Organic and Biological Chemistry (LibreTexts) by David W. Ball, John W. Hill, and Rhonda J. Scott, licensed under CC BY-NC-SA 3.0
Reference
Hein, M., Pattison, S., Arena, S., & Best, L. (2013). Introduction to general, organic, and biochemistry (11th ed.). John Wiley & Sons, Inc.

