5.3 Subatomic Particles of the Atom
Learning Objectives
By the end of this section, you will be able to:
- Outline milestones in the development of modern atomic theory
- Summarize and interpret the results of the experiments of Thomson, Millikan, and Rutherford
- Describe the three subatomic particles that compose atoms
Scientists have made significant progress in furthering our understanding of atomic theory. Much of this came from the results of several seminal experiments that revealed the details of the internal structure of atoms. Here, we will discuss some of those key developments, with an emphasis on the application of the scientific method, as well as understanding how the experimental evidence was analyzed. While the historical persons and dates behind these experiments can be quite interesting, it is most important to understand the concepts resulting from their work.
Atomic Theory after the Nineteenth Century
If the matter were composed of atoms, what were atoms composed of? Was it just predominately European scientists questioning the properties of matter, its constituents, its behaviour, and why and how it exists? Or at that time, were other groups of people around the world, such as Indigenous communities, also asking similar questions? From their experiences, traditions, and cultural practices did they test their own hypotheses about the different matter around them - what makes mud, mud, and why is it different from sweetgrass before and after it is burned? Did they, too, ask the question what makes the smallest particles, or is there something smaller? Matter is made up of inconceivably small atoms, and yet scientists found atoms contain even smaller subatomic particles, including electrons, protons, and neutrons. The discovery of these subatomic particles is discussed next.
Revelation of the Electron
In the late 1800s, a number of scientists interested in questions like these investigated the electrical discharges that could be produced in low-pressure gases, with the most significant discovery made in 1897 by English physicist J. J. Thomson using a cathode ray tube. This apparatus consisted of a sealed glass tube from which almost all the air had been removed; the tube contained two metal electrodes. When high voltage was applied across the electrodes, a visible beam called a cathode ray appeared between them. This beam was deflected toward the positive charge and away from the negative charge, and was produced in the same way with identical properties when different metals were used for the electrodes. In similar experiments, the ray was simultaneously deflected by an applied magnetic field, and measurements of the extent of deflection and the magnetic field strength allowed Thomson to calculate the charge-to-mass ratio of the cathode ray particles. The results of these measurements indicated that these particles were much lighter than atoms (Figure 5.3a).
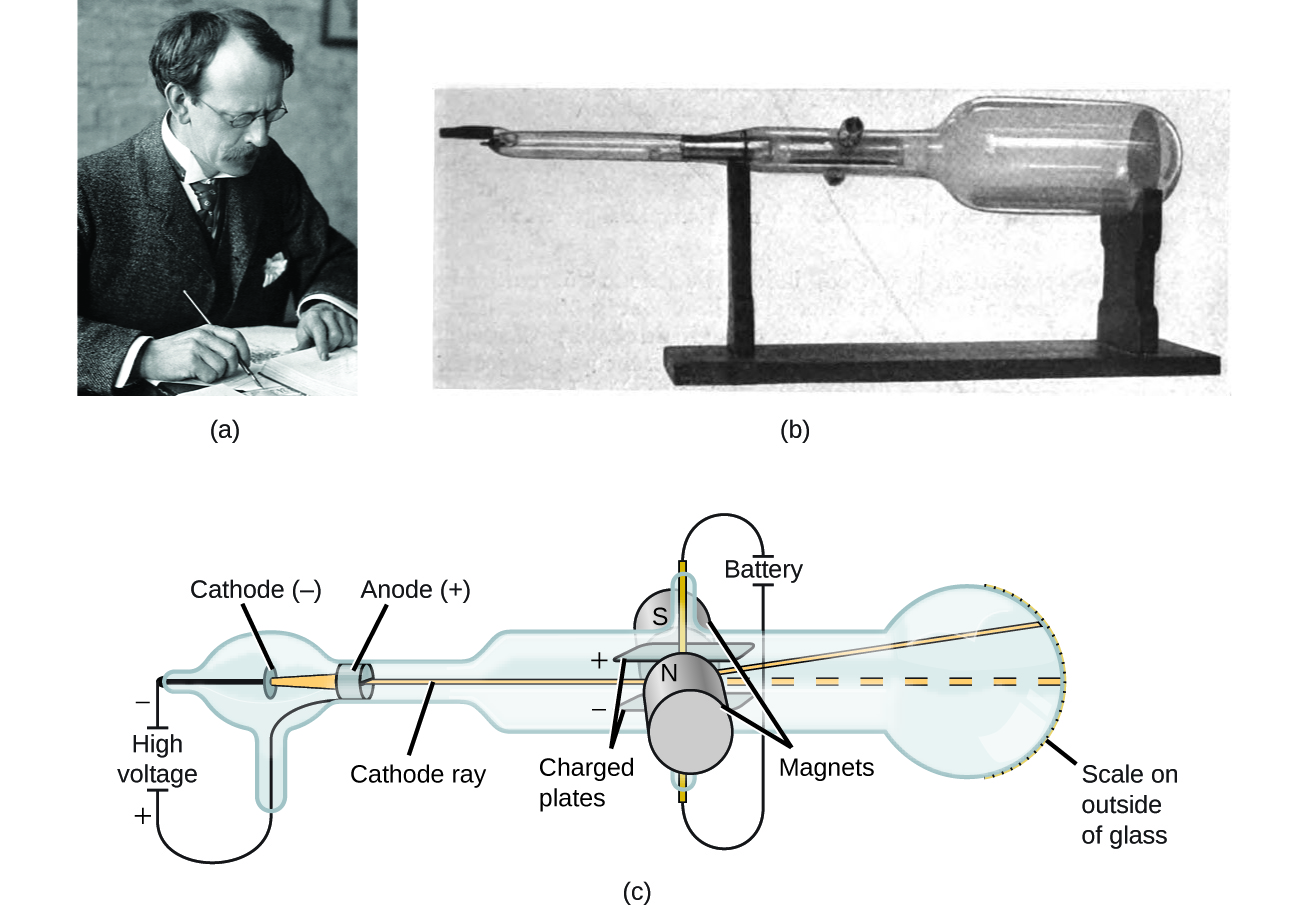
Based on his observations, here is what Thomson proposed and why:
- The particles are attracted by positive (+) charges and repelled by negative (−) charges, so the particles of the cathode ray must be negatively charged since like charges repel and unlike charges attract.
- they are less massive than atoms and indistinguishable, regardless of the source material, so they must be fundamental, subatomic constituents of all atoms.
Although controversial at the time, Thomson’s idea was gradually accepted, and his cathode ray particle is what we now call an electron, a negatively charged, subatomic particle with a mass more than one thousand-times less that of an atom. The term “electron” was initially coined in 1891 by Irish physicist George Stoney, meaning “electric ion.” Stoney originally recognized the atom must have a unit of electricity and charge associated with the atom, but he had no experimental proof. It was J.J Thomson who took this theory and orchestrated scientific experiments using the cathode rays to prove atoms contained charged particles - the fundamental unit of charge - the electron.
J.J. Thomson Talks About the Size of the Electron
Listen to Thomson describe his discovery in his own voice.
In 1909, more information about the electron was uncovered by American physicist Robert A. Millikan via his “oil drop” experiments. Millikan created microscopic oil droplets, which could be electrically charged by friction as they formed or by using X-rays. These droplets initially fell due to gravity, but their downward progress could be slowed or even reversed by an electric field lower in the apparatus. By adjusting the electric field strength and making careful measurements and appropriate calculations, Millikan was able to determine the charge on individual drops (Figure 5.3b).
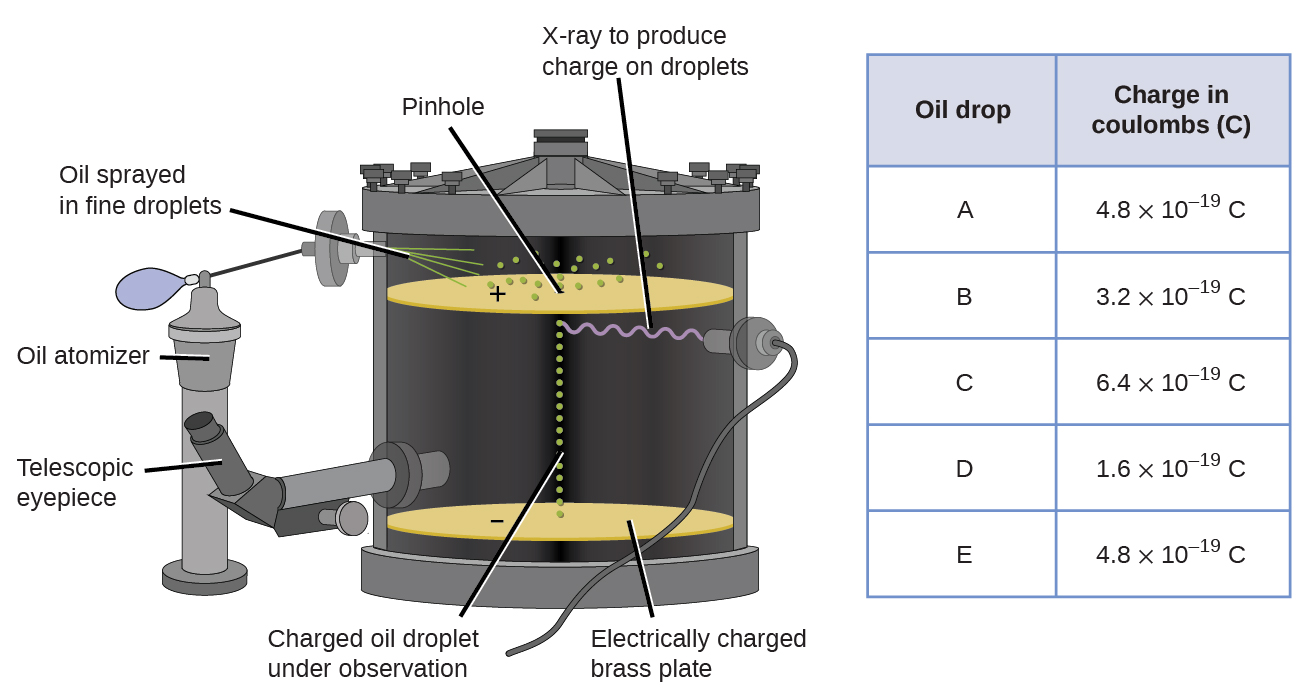
Looking at the charge data that Millikan gathered, you may have recognized that the charge of an oil droplet is always a multiple of a specific charge, 1.6 × 10−19 C. The symbol C - the coulomb - is the unit of electric charge in the International System of Units (SI). Millikan concluded that this electric charge value must therefore be a fundamental charge - the charge of a single electron - with his measured charges due to an excess of one electron (1 times 1.6 × 10−19 C), two electrons (2 times 1.6 × 10−19 C), three electrons (3 times 1.6 × 10−19 C), and so on, on a given oil droplet. Since the charge of an electron was now known due to Millikan’s research, and the charge-to-mass ratio was already known due to Thomson’s research (1.759 × 1011 C/kg), it only required a simple calculation to determine the mass of the electron as well.
A summary of the important concepts discovered about electrons include:
- They are negatively charged subatomic particles
- Mass of the electron is found to be 9.110 x 10-28 g or 9.110 x 10-31 kg
- The charge on the electron is -1.602 x 10-19 coulombs
- when considering the chemical behaviour of subatomic particles in an atom and its ability to form ions, it is customary to consider these particles as having a relative charge: An electron carries a negative charge (-1) and is represented by the symbol, e-.
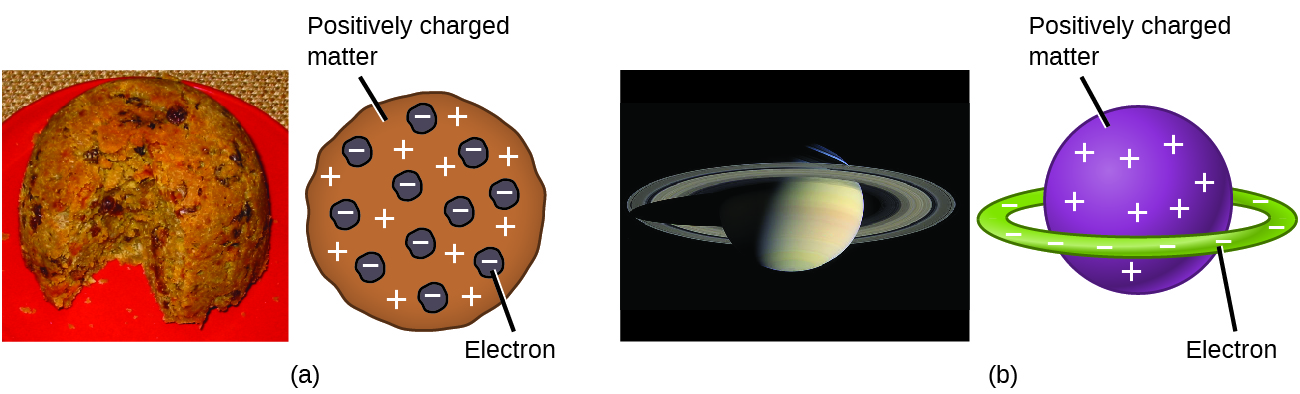
Scientists had now established that the atom was not indivisible as Dalton had believed, but consisted of smaller subatomic charged particles. Due to the work of Thomson, Millikan, and others, the charge and mass of the negative, subatomic particles—the electrons—were known. However, the positively charged part of an atom was not yet well understood. It is suggested that the proton was observed by Eugen Goldstein in 1886 when he used anode rays of a hydrogen ion. However, it was the later experiments of J.J. Thomson and Ernest Rutherford that uncovered the nature of the positively charged proton. In 1904, Thomson proposed the “plum pudding” model of atoms, which described a positively charged mass with an equal amount of negative charge in the form of electrons embedded in it, since all atoms are electrically neutral. A competing model had been proposed in 1903 by Hantaro Nagaoka, who postulated a Saturn-like atom, consisting of a positively charged sphere surrounded by a halo of electrons (Figure 5.3c)
Revelation of the Proton
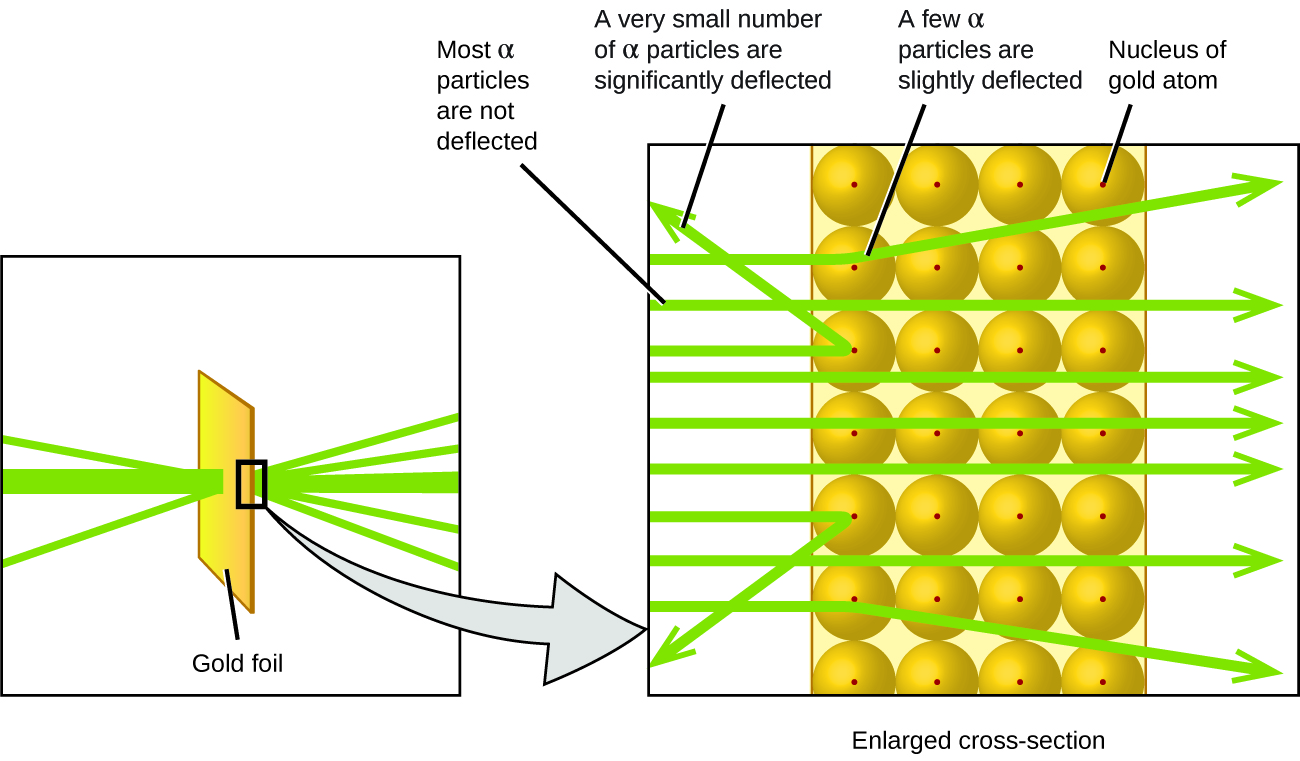
The next major development in understanding the atom came from Ernest Rutherford, a physicist from New Zealand who largely spent his scientific career in Canada and England. He performed a series of experiments using a beam of high-speed, positively charged alpha particles (α particles) that were produced by the radioactive decay of radium; α particles consist of two protons and two neutrons (you will learn more about radioactive decay if you study nuclear chemistry). Rutherford and his colleagues Hans Geiger (later famous for the Geiger counter) and Ernest Marsden aimed a beam of α particles, the source of which was embedded in a lead block to absorb most of the radiation, at a very thin piece of gold foil and examined the resultant scattering of the α particles using a luminescent screen that glowed briefly where hit by an α particle.
What did they discover? Most particles passed right through the foil without being deflected at all. However, some were diverted slightly, and a very small number were deflected almost straight back toward the source (Figure 5.3d). Rutherford described finding these results: “It was quite the most incredible event that has ever happened to me in my life. It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you” [1]
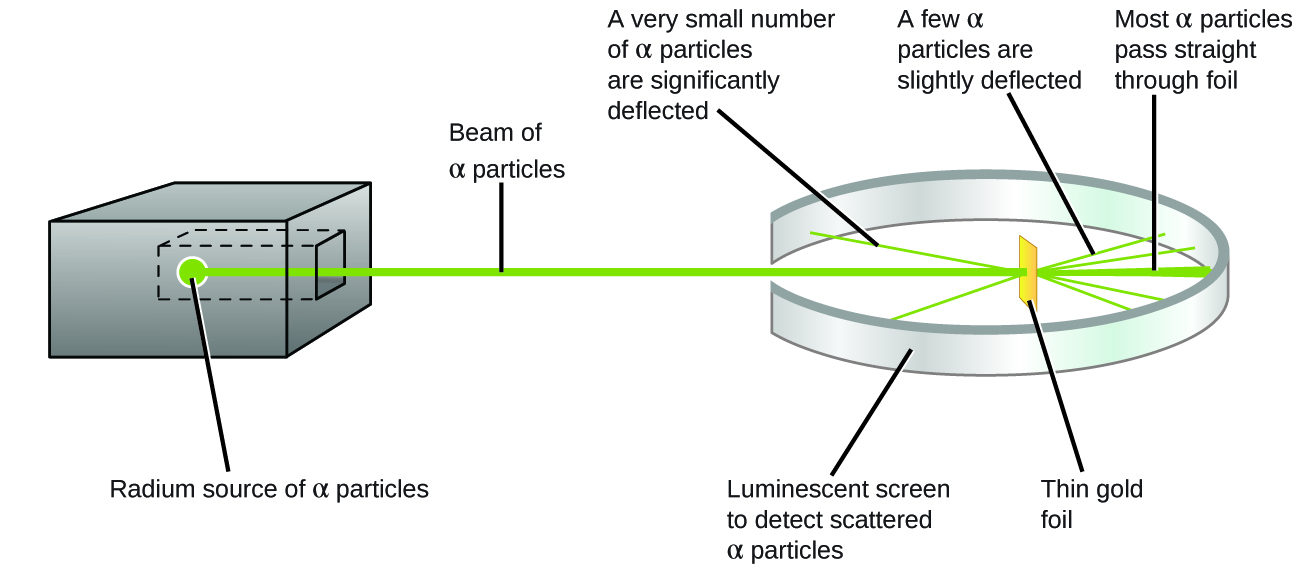
Here is what Rutherford deduced: Because most of the fast-moving α particles passed through the gold atoms undeflected, they must have traveled through essentially empty space inside the atom. Alpha particles are positively charged, so deflections arose when they encountered another positive charge. Since like charges repel one another, the few positively charged α particles that changed paths abruptly must have hit, or closely approached, another body that also had a highly concentrated, positive charge. Since the deflections occurred a small fraction of the time, this charge only occupied a small amount of the space in the gold foil. Analyzing a series of such experiments in detail, Rutherford drew two conclusions:
- The volume occupied by an atom must consist of a large amount of empty space.
- A small, relatively heavy, positively charged body, termed the nucleus, must be at the centre of each atom.
The Rutherford Experiment
This simulation of the Rutherford gold foil experiment allows you to adjust the slit width to produce a narrower or broader beam of α particles to see how that affects the scattering pattern.
This analysis led Rutherford to propose a model in which an atom consists of a very small, positively charged nucleus, in which most of the mass of the atom is concentrated, surrounded by the negatively charged electrons, so that the atom is electrically neutral (Figure 5.3e). After many more experiments, Rutherford also discovered that the nuclei of other elements contain the hydrogen nucleus as a “building block,” and he named this more fundamental particle the proton, the positively charged, subatomic particle found in the nucleus. With one addition, which you will learn next, this nuclear model of the atom, proposed over a century ago, is still used today.
Exercise 5.3a
Investigate the differences between a “plum pudding” atom and a Rutherford atom by firing α particles at each type of atom using the following PhET simulation: Rutherford Scattering
Activity source: Simulation by PhET Interactive Simulations, University of Colorado Boulder, licensed under CC BY-4.0
A summary of the important concepts discovered about protons include:
- Protons are positively charged subatomic particles
- Mass of the proton is found to be 1.673 x 10-24 g, which is 1,836 times the mass of an electron.
- Protons are found in the central part of an atom called the nucleus.
- The charge on the proton is +1.602 x 10-19 coulombs
- When considering the chemical behaviour of subatomic particles in an atom and its ability to form ions, it is customary to consider these particles as having a relative charge: A proton carries a positive charge (+1) and is represented by the symbol p+. As noted earlier, an electron carries a negative charge (-1) and is represented by the symbol, e-. In a neutral atom, the number of protons equals the number of electrons, which means atoms of a given element are neutral.
The Neutron
One puzzle remained with regard to the subatomic particles of an atom. The nucleus of an atom was known to contain almost all of the mass of an atom, but the number of protons was only providing half, or less, of that atom's mass. Therefore, there must be some other type of subatomic matter present in the nucleus that had yet to be discovered. Different proposals were made to explain what constituted the remaining mass, while still maintaining the neutral charge of the atom - the existence of neutral particles in the nucleus. As you might expect, detecting uncharged particles is very challenging, and it was not until 1932 that James Chadwick found evidence of neutrons, uncharged, subatomic particles with a mass approximately the same as that of protons, 1.67493 × 10−24 g. Neutrons, having no relative electric charge and are represented by the symbol n0. The existence of the neutron also explained isotopes, alternative forms of a given element. Isotopes of a given element differ in mass because they have different numbers of neutrons, but they are chemically identical because they have the same number of protons. The concept of isotopes will be explained in more detail later in this chapter.
Watch Subatomic Particles Explained in Under 4 Minutes (3 mins 39 s)
Exercise 5.3b
Check Your Learning Exercise (Text Version)
Review the scientist name list below. Match each of the seven scientists with their key discovery by filling in the [BLANK] with the correct scientist’s name.
Scientist Name List (includes 7 names):
Democritus, Aristotle, John Dalton, J.J Thomson, Robert Millikan, Ernst Rutherford, James Chadwick
QUESTIONS:
- The scientist who concluded that matter is composed of tiny, indivisible atoms that combine, separate, and rearrange in whole number ratios to form new matter is [BLANK].
- [BLANK] is the scientist who postulated the nuclear model of the atom; the nuclear atom is mostly empty space with nearly all of its mass concentrated in the tiny central positively charged nucleus, which is surrounded by negatively charged electrons.
- The scientist who hypothesized that all matter was composed of small, finite particles that they called atomos, meaning “indivisible” is [BLANK].
- [BLANK] identified the mass of an electron to be 9.109x10^-31 kilograms
- [BLANK] suggested a philosophical concept of matter such that it consisted of four elements – fire, earth, air, and water – and could be infinitely divided
- This scientist, [BLANK], discovered the neutron, a subatomic particle with no charge and is in the tiny nucleus of an atom.
- [BLANK] performed experiments with cathode ray tubes and discovered that all atoms contain tiny negatively charged subatomic particles and called them electrons. This scientist proposed the plum pudding model of the atom, which had a uniform sphere of positive charge with negatively charged electrons embedded within the sphere.
Check Your Answer[2]
Source: "Exercise 5.3b" by Jackie MacDonald, licensed under CC BY-NC-SA 4.0
Attribution & References
Except where otherwise noted, this page is adapted by Jackie MacDonald from “Evolution of Atomic Theory” In Chemistry 2e (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson is licensed under CC BY 4.0. Access for free at Chemistry 2e (OpenStax).
- Ernest Rutherford, “The Development of the Theory of Atomic Structure,” ed. J. A. Ratcliffe, in Background to Modern Science, eds. Joseph Needham and Walter Pagel, (Cambridge, UK: Cambridge University Press, 1938), 61–74. Accessed September 22, 2014, https://ia600508.us.archive.org/3/items/backgroundtomode032734mbp/backgroundtomode032734mbp.pdf. (p. 68). ↵
- (1) John Dalton; (2) Ernst Rutherford; (3) Democritus; (4) Robert Millikan; (5) Aristotle; (6) James Chadwick; (7) J.J Thomson ↵
negatively charged, subatomic particle of relatively low mass located outside the nucleus
positively charged particle consisting of two protons and two neutrons
massive, positively charged center of an atom made up of protons and neutrons
positively charged, subatomic particle located in the nucleus
uncharged, subatomic particle located in the nucleus

