3.1 Elements
Learning Objectives
By the end of this section, you will be able to:
- Write and interpret symbols that depict the element
Chemical Symbols
A chemical symbol is an abbreviation that we use to indicate an element or an atom of an element. For example, the symbol for mercury is Hg (Figure 3.1a). We use the same symbol to indicate one atom of mercury (microscopic domain) or to label a container of many atoms of the element mercury (macroscopic domain).

The symbols for several common elements and their atoms are listed in Table 3.1a Some symbols are derived from the common name of the element; others are abbreviations of the name in another language. Most symbols have one or two letters, but three-letter symbols have been used to describe some elements that have atomic numbers greater than 112. To avoid confusion with other notations, only the first letter of a symbol is capitalized. For example, Co is the symbol for the element cobalt, but CO is the notation for the compound carbon monoxide, which contains atoms of the elements carbon (C) and oxygen (O). All known elements and their symbols are in the periodic table (Figure 3.1c).
| Element | Symbol | Element | Symbol |
|---|---|---|---|
| aluminum | Al | iron | Fe (from ferrum) |
| bromine | Br | lead | Pb (from plumbum) |
| calcium | Ca | magnesium | Mg |
| carbon | C | mercury | Hg (from hydrargyrum) |
| chlorine | Cl | nitrogen | N |
| chromium | Cr | oxygen | O |
| cobalt | Co | potassium | K (from kalium) |
| copper | Cu (from cuprum) | silicon | Si |
| fluorine | F | silver | Ag (from argentum) |
| gold | Au (from aurum) | sodium | Na (from natrium) |
| helium | He | sulfur | S |
| hydrogen | H | tin | Sn (from stannum) |
| iodine | I | zinc | Zn |
Traditionally, the discoverer (or discoverers) of a new element names the element. However, until the name is recognized by the International Union of Pure and Applied Chemistry (IUPAC), the recommended name of the new element is based on the Latin word(s) for its atomic number. For example, element 106 was called unnilhexium (Unh), element 107 was called unnilseptium (Uns), and element 108 was called unniloctium (Uno) for several years. These elements are now named after scientists (or occasionally locations); for example, element 106 is now known as seaborgium (Sg) in honour of Glenn Seaborg, a Nobel Prize winner who was active in the discovery of several heavy elements.
Scientists in Action: Marguerite Perey, PhD.
The discovery of the element 87 on the periodic table is thanks to Marguerite Perey, a French woman scientist. The story of the discovery of element 87, known as Francium, can be found on the Royal Society of Chemistry [New Tab] website. Marguerite Perey was nominated five times for a Nobel Prize but never received one.
Source: Chapman, K. (2020, August 3). Marguerite Perey and the last element in nature. Chemistry World. https://www.chemistryworld.com/culture/marguerite-perey-and-the-last-element-in-nature/4012198.article
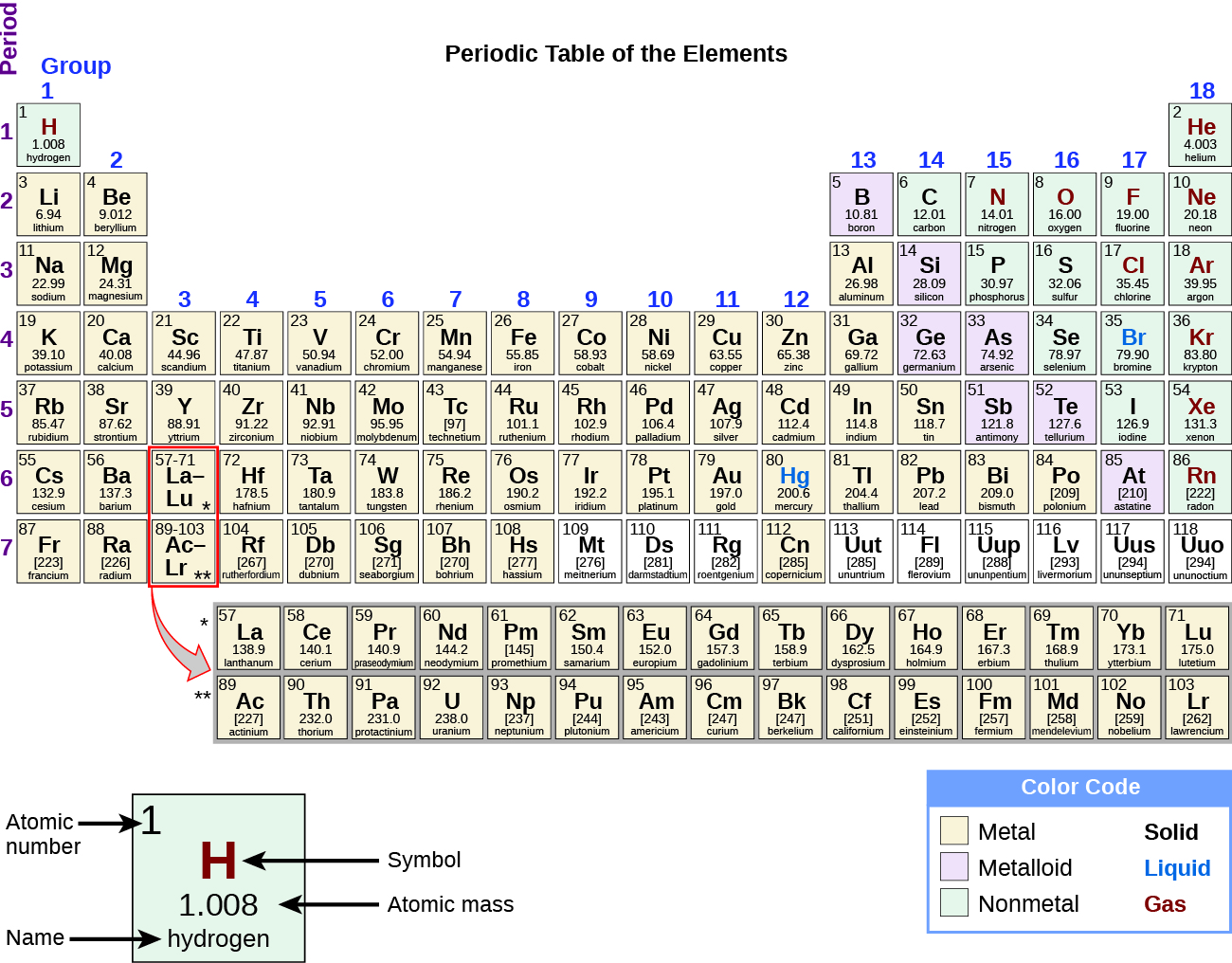
Exercise 3.1a: Drag the element names to their proper symbol on the periodic table
Links to Interactive Learning Tools
Visit the IUPAC, the International Union of Pure and Applied Chemistry website to learn more about IUPAC, and explore its periodic table.
Practice Name That Element from the Physics Classroom.
Attribution & References
Except where otherwise noted, this page is adapted by Adrienne Richards from “2.3 Atomic Structure and Symbolism” In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0.
- (a) Mg; (b) Cu; (c) Cl; (d) Au; (e) Si; (f) K; (g) Fe; (h) W; ↵
one-, two-, or three-letter abbreviation used to represent an element or its atoms

