18.6 Corrosion
Learning Objectives
- Define corrosion
- List some of the methods used to prevent or slow corrosion
Corrosion is usually defined as the degradation of metals due to an electrochemical process. The formation of rust on iron, tarnish on silver, and the blue-green patina that develops on copper are all examples of corrosion. The total cost of corrosion in the United States is significant, with estimates in excess of half a trillion dollars a year.
Statue of Liberty: Changing Colours
The Statue of Liberty is a landmark every American recognizes. The Statue of Liberty is easily identified by its height, stance, and unique blue-green colour (Figure 18.6a). When this statue was first delivered from France, its appearance was not green. It was brown, the colour of its copper “skin.” So how did the Statue of Liberty change colours? The change in appearance was a direct result of corrosion. The copper that is the primary component of the statue slowly underwent oxidation from the air. The oxidation-reduction reactions of copper metal in the environment occurs in several steps. Copper metal is oxidized to copper(I) oxide (Cu2O), which is red, and then to copper(II) oxide, which is black
[latex]\begin{array}{r @{{}\longrightarrow{}} ll} 2\text{ Cu}(s)\;+\;\frac{1}{2}\text{ O}_2(g) & \longrightarrow \text{Cu}_2\text{O}(s) & (\text{red}) \\[0.5em] \text{Cu}_2\text{O}(s)\;+\;\frac{1}{2}\text{ O}_2(g) & \longrightarrow 2\text{ CuO}(s) & (\text{black}) \end{array}[/latex]
Coal, which was often high in sulfur, was burned extensively in the early part of the last century. As a result, sulfur trioxide, carbon dioxide, and water all reacted with the CuO
[latex]\begin{array}{rl @{{}\longrightarrow{}} ll} 2\text{ CuO}(s)\;+\;\text{CO}_2(g)\;+\;\text{H}_2\text{O}(l) & \longrightarrow \text{Cu}_2\text{CO}_3(\text{OH})_2(s) & (\text{green}) \\[0.5em] 3\text{ CuO}(s)\;+\;2\text{ CO}_2(g)\;+\;\text{H}_2\text{O}(l) & \longrightarrow \text{Cu}_2(\text{CO}_3)_2(\text{OH})_2(s) & (\text{blue}) \\[0.5em] 4\text{ CuO}(s)\;+\;\text{SO}_3(g)\;+\;3\text{ H}_2\text{O}(l) & \longrightarrow \text{Cu}_4\text{SO}_4(\text{OH})_6(s) & (\text{green}) \end{array}[/latex]
These three compounds are responsible for the characteristic blue-green patina seen today. Fortunately, formation of the patina created a protective layer on the surface, preventing further corrosion of the copper skin. The formation of the protective layer is a form of passivation, which is discussed further in a later chapter.

Perhaps the most familiar example of corrosion is the formation of rust on iron. Iron will rust when it is exposed to oxygen and water. The main steps in the rusting of iron appear to involve the following (Figure 18.6b). Once exposed to the atmosphere, iron rapidly oxidizes.
The electrons reduce oxygen in the air in acidic solutions.
What we call rust is hydrated iron(III) oxide, which forms when iron(II) ions react further with oxygen.
The number of water molecules is variable, so it is represented by x. Unlike the patina on copper, the formation of rust does not create a protective layer and so corrosion of the iron continues as the rust flakes off and exposes fresh iron to the atmosphere.
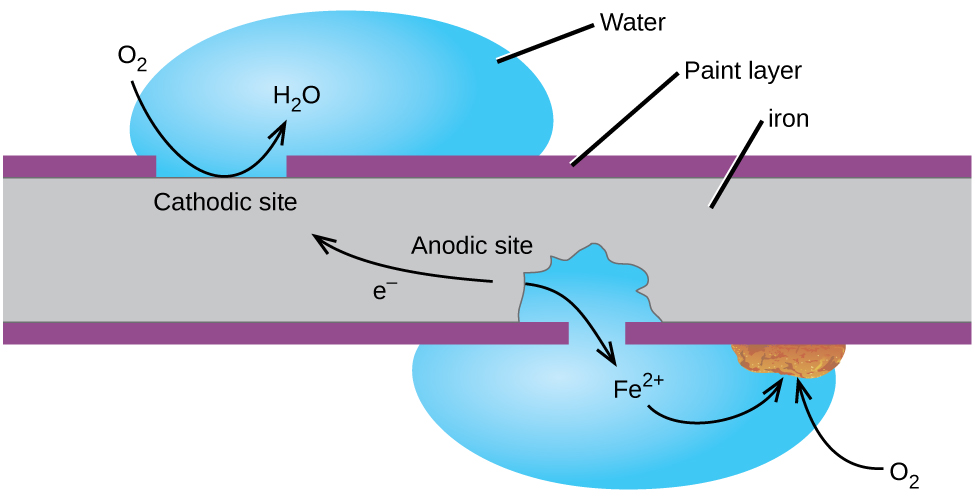
One way to keep iron from corroding is to keep it painted. The layer of paint prevents the water and oxygen necessary for rust formation from coming into contact with the iron. As long as the paint remains intact, the iron is protected from corrosion.
Other strategies include alloying the iron with other metals. For example, stainless steel is mostly iron with a bit of chromium. The chromium tends to collect near the surface, where it forms an oxide layer that protects the iron.
Zinc-plated or galvanized iron uses a different strategy. Zinc is more easily oxidized than iron because zinc has a lower reduction potential. Since zinc has a lower reduction potential, it is a more active metal. Thus, even if the zinc coating is scratched, the zinc will still oxidize before the iron. This suggests that this approach should work with other active metals.
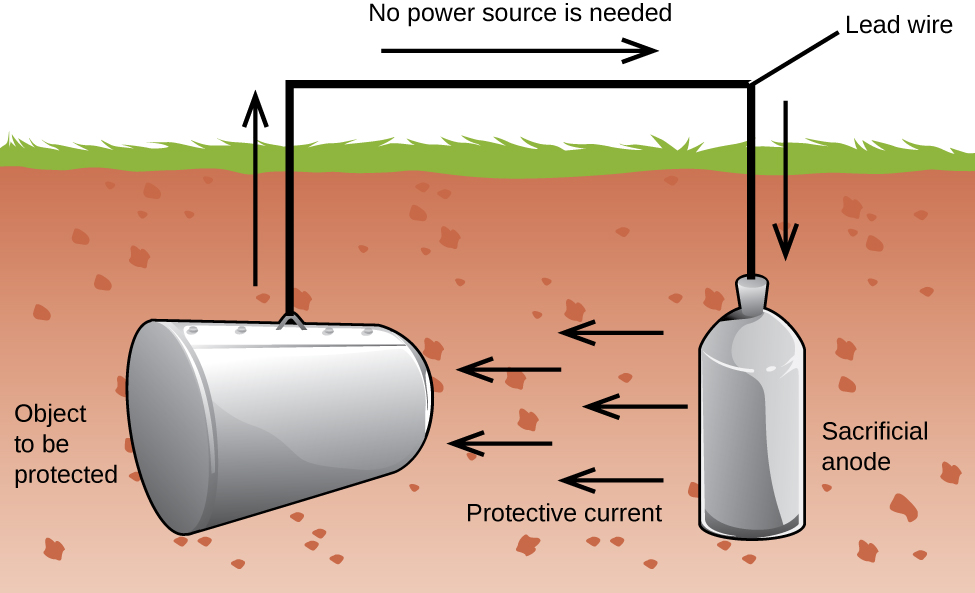
Another important way to protect metal is to make it the cathode in a galvanic cell. This is cathodic protection and can be used for metals other than just iron. For example, the rusting of underground iron storage tanks and pipes can be prevented or greatly reduced by connecting them to a more active metal such as zinc or magnesium (Figure 18.6c). This is also used to protect the metal parts in water heaters. The more active metals (lower reduction potential) are called sacrificial anodes because as they get used up as they corrode (oxidize) at the anode. The metal being protected serves as the cathode, and so does not oxidize (corrode). When the anodes are properly monitored and periodically replaced, the useful lifetime of the iron storage tank can be greatly extended.
Attribution & References
Except where otherwise noted, this page is adapted by David Wegman from "17.6 Corrosion" In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax).
degradation of metal through an electrochemical process
method for protecting iron by covering it with zinc, which will oxidize before the iron; zinc-plated iron
method of protecting metal by using a sacrificial anode and effectively making the metal that needs protecting the cathode, thus preventing its oxidation
more active, inexpensive metal used as the anode in cathodic protection; frequently made from magnesium or zinc

