1.1 Chemistry in Context
Learning Objectives
By the end of this section, you will be able to:
- Outline the historical development of chemistry
- Provide examples of the importance of chemistry in everyday life
- Describe the scientific method
- Differentiate among hypotheses, theories, and laws
- Provide examples illustrating macroscopic, microscopic, and symbolic domains
Throughout human history, people have tried to convert matter into more useful forms. Our Stone Age ancestors chipped pieces of flint into useful tools and carved wood into statues and toys. These endeavours involved changing the shape of a substance without changing the substance itself. But as our knowledge increased, humans began to change the composition of the substances as well—clay was converted into pottery, hides were cured to make garments, copper ores were transformed into copper tools and weapons, and grain was made into bread.
Humans began to practice chemistry when they learned to control fire and use it to cook, make pottery, and smelt metals. Subsequently, they began to separate and use specific components of matter. A variety of drugs such as aloe, myrrh, and opium were isolated from plants. Dyes, such as indigo and Tyrian purple, were extracted from plant and animal matter. Metals were combined to form alloys—for example, copper and tin were mixed together to make bronze—and more elaborate smelting techniques produced iron. Alkalis were extracted from ashes, and soaps were prepared by combining these alkalis with fats. Alcohol was produced by fermentation and purified by distillation.
Attempts to understand the behaviour of matter extend back for more than 2500 years. As early as the sixth century BC, Greek philosophers discussed a system in which water was the basis of all things. You may have heard of the Greek postulate that matter consists of four elements: earth, air, fire, and water. Subsequently, an amalgamation of chemical technologies and philosophical speculations were spread from Egypt, China, and the eastern Mediterranean by alchemists, who endeavoured to transform “base metals” such as lead into “noble metals” like gold, and to create elixirs to cure disease and extend life (Figure 1.1.a).

From alchemy came the historical progressions that led to modern chemistry: the isolation of drugs from natural sources, metallurgy, and the dye industry. Today, chemistry continues to deepen our understanding and improve our ability to harness and control the behaviour of matter. This effort has been so successful that many people do not realize either the central position of chemistry among the sciences or the importance and universality of chemistry in daily life.
Chemistry: The Central Science
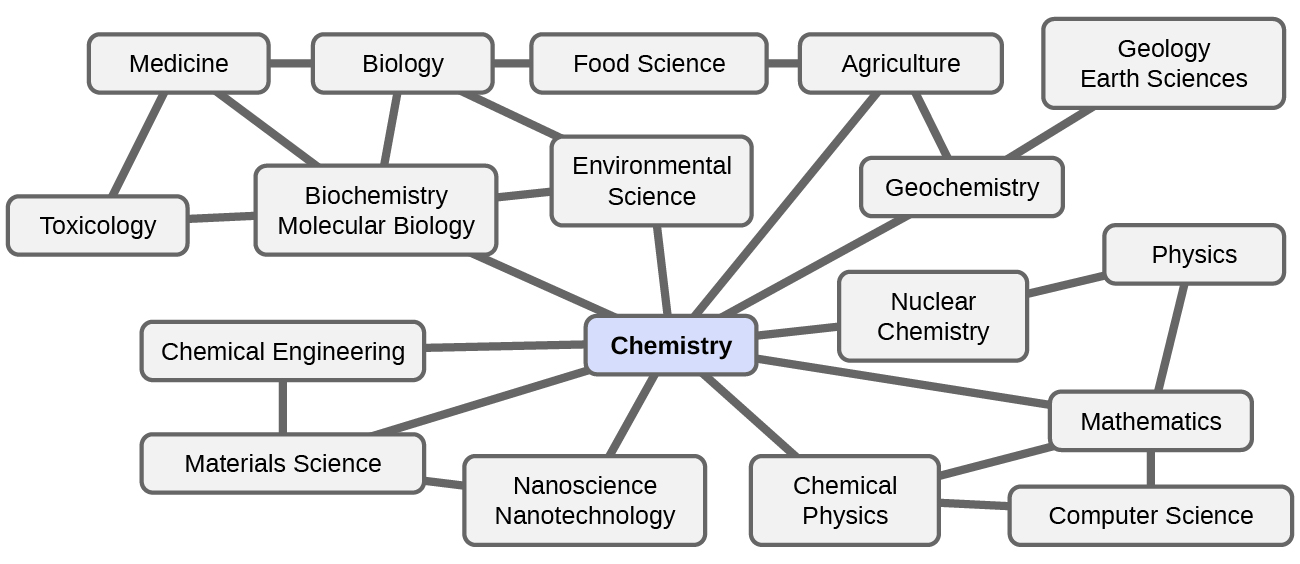
Chemistry is sometimes referred to as “the central science” due to its interconnectedness with a vast array of other STEM disciplines (STEM stands for areas of study in the science, technology, engineering, and math fields). Chemistry and the language of chemists play vital roles in biology, medicine, materials science, forensics, environmental science, and many other fields (Figure 1.1b). The basic principles of physics are essential for understanding many aspects of chemistry, and there is extensive overlap between many subdisciplines within the two fields, such as chemical physics and nuclear chemistry. Mathematics, computer science, and information theory provide important tools that help us calculate, interpret, describe, and generally make sense of the chemical world. Biology and chemistry converge in biochemistry, which is crucial to understanding the many complex factors and processes that keep living organisms (such as us) alive. Chemical engineering, materials science, and nanotechnology combine chemical principles and empirical findings to produce useful substances, ranging from gasoline to fabrics to electronics. Agriculture, food science, veterinary science, and brewing and wine making help provide sustenance in the form of food and drink to the world’s population. Medicine, pharmacology, biotechnology, and botany identify and produce substances that help keep us healthy. Environmental science, geology, oceanography, and atmospheric science incorporate many chemical ideas to help us better understand and protect our physical world. Chemical ideas are used to help understand the universe in astronomy and cosmology.
What are some changes in matter that are essential to daily life? Digesting and assimilating food, synthesizing polymers that are used to make clothing, containers, cookware, and credit cards, and refining crude oil into gasoline and other products are just a few examples. As you proceed through this course, you will discover many different examples of changes in the composition and structure of matter, how to classify these changes and how they occurred, their causes, the changes in energy that accompany them, and the principles and laws involved. As you learn about these things, you will be learning chemistry, the study of the composition, properties, and interactions of matter. The practice of chemistry is not limited to chemistry books or laboratories: It happens whenever someone is involved in changes in matter or in conditions that may lead to such changes.
The Scientific Method
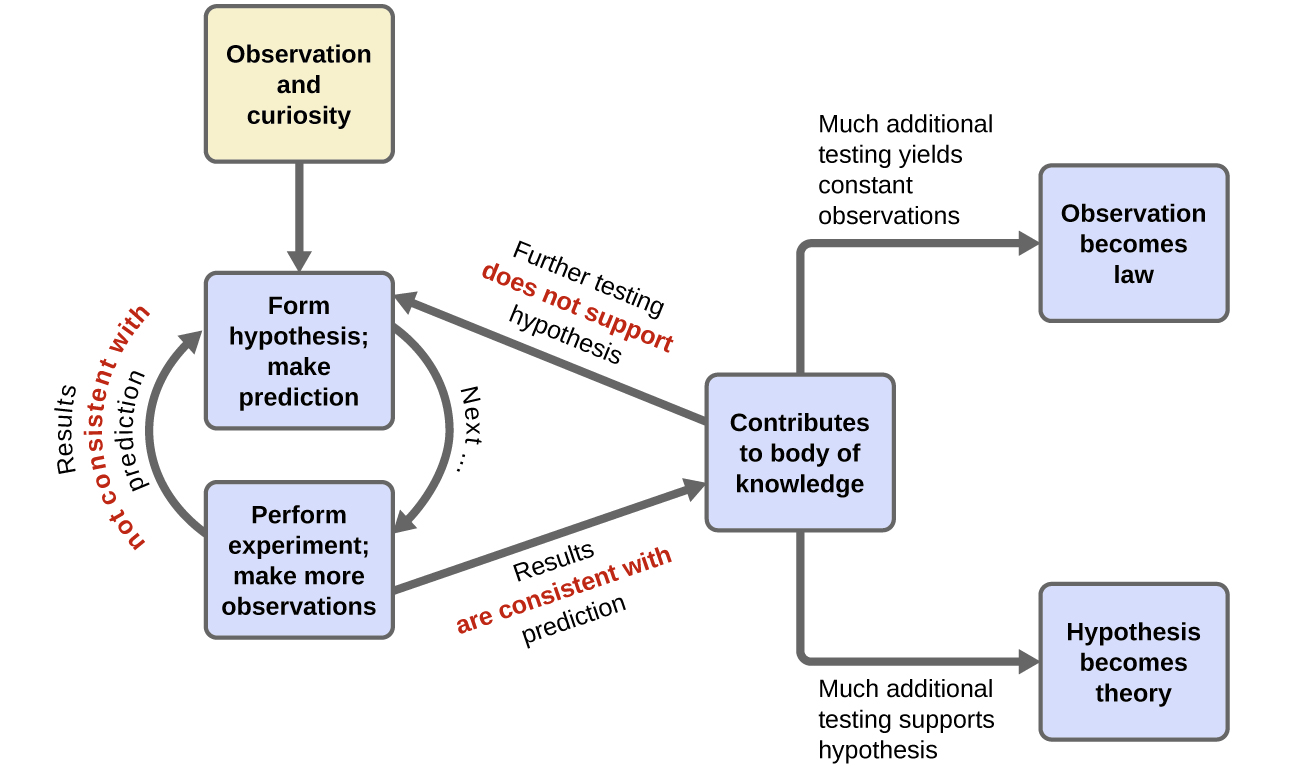
Chemistry is a science based on observation and experimentation. Doing chemistry involves attempting to answer questions and explain observations in terms of the laws and theories of chemistry, using procedures that are accepted by the scientific community. There is no single route to answering a question or explaining an observation, but there is an aspect common to every approach: Each uses knowledge based on experiments that can be reproduced to verify the results. Some routes involve a hypothesis, a tentative explanation of observations that acts as a guide for gathering and checking information. We test a hypothesis by experimentation, calculation, and/or comparison with the experiments of others and then refine it as needed.
Some hypotheses are attempts to explain the behaviour that is summarized in laws. The laws of science summarize a vast number of experimental observations, and describe or predict some facet of the natural world. If such a hypothesis turns out to be capable of explaining a large body of experimental data, it can reach the status of a theory. Scientific theories are well-substantiated, comprehensive, testable explanations of particular aspects of nature. Theories are accepted because they provide satisfactory explanations, but they can be modified if new data become available. The path of discovery that leads from question and observation to law or hypothesis to theory, combined with experimental verification of the hypothesis and any necessary modification of the theory, is called the scientific method (Figure 1.1c).

Scientists in Action: George Washington Carver, PhD.
George Washington Carver was born into slavery in Missouri. His interest in science started with taking care of plants at a young age. He is most famous for his contributions to agricultural chemistry, and he is credited with developing over 100 uses for the peanut. He was the first African American to have a national monument dedicated to him. The American Chemical Society dedicated his work as a National Historic Chemical Landmark in 2005.Learn more about Dr. George Washington Carver in this American Chemical Society Commemorative booklet [New Tab][PDF]. He was dedicated to the continuing education of poor farmers and took his Jessup wagon (think of it as a traveling lab) around to rural communities to share what he had learned. His epitaph reads “He could have added fortune to fame, but caring for neither, he found happiness and honour in being helpful to the world.” If you’d like to, listen to an old audio recording of George Washington Carver [New Tab].
The Domains of Chemistry
Chemists study and describe the behaviour of matter and energy in three different domains: macroscopic, microscopic, and symbolic. These domains provide different ways of considering and describing chemical behaviour.
Macro is a Greek word that means “large.” The macroscopic domain is familiar to us: It is the realm of everyday things that are large enough to be sensed directly by human sight or touch. In daily life, this includes the food you eat and the breeze you feel on your face. The macroscopic domain includes every day and laboratory chemistry, where we observe and measure physical and chemical properties, or changes such as density, solubility, and flammability.
The microscopic domain of chemistry is almost always visited in the imagination. Micro also comes from Greek and means “small.” Some aspects of the microscopic domains are visible through a microscope, such as a magnified image of graphite or bacteria. Viruses, for instance, are too small to be seen with the naked eye, but when we’re suffering from a cold, we’re reminded of how real they are.
However, most of the subjects in the microscopic domain of chemistry—such as atoms and molecules—are too small to be seen even with standard microscopes and often must be pictured in the mind. Other components of the microscopic domain include ions and electrons, protons and neutrons, and chemical bonds, each of which is far too small to see. This domain includes the individual metal atoms in a wire, the ions that compose a salt crystal, the changes in individual molecules that result in a colour change, the conversion of nutrient molecules into tissue and energy, and the evolution of heat as bonds that hold atoms together are created.
The symbolic domain contains the specialized language used to represent components of the macroscopic and microscopic domains. Chemical symbols (such as those used in the periodic table), chemical formulas, and chemical equations are part of the symbolic domain, as are graphs and drawings. We can also consider calculations as part of the symbolic domain. These symbols play an important role in chemistry because they help interpret the behaviour of the macroscopic domain in terms of the components of the microscopic domain. One of the challenges for students learning chemistry is recognizing that the same symbols can represent different things in the macroscopic and microscopic domains, and one of the features that makes chemistry fascinating is the use of a domain that must be imagined to explain behaviour in a domain that can be observed.
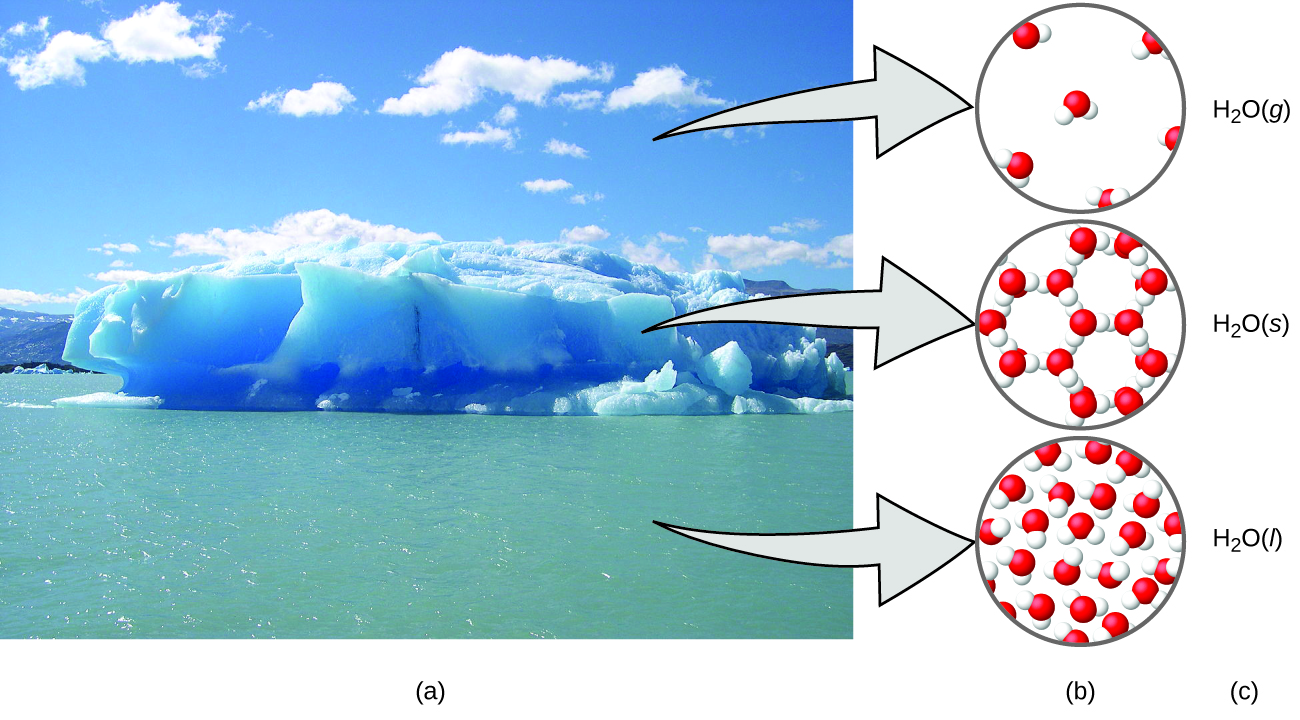
A helpful way to understand the three domains is via the essential and ubiquitous substance of water. That water is a liquid at moderate temperatures, will freeze to form a solid at lower temperatures, and boil to form a gas at higher temperatures (Figure 1.1e) are macroscopic observations. But some properties of water fall into the microscopic domain—what we cannot observe with the naked eye. The description of water as comprised of two hydrogen atoms and one oxygen atom, and the explanation of freezing and boiling in terms of attractions between these molecules, is within the microscopic arena. The formula H2O, which can describe water at either the macroscopic or microscopic levels, is an example of the symbolic domain. The abbreviations (g) for gas, (s) for solid, and (l) for liquid are also symbolic.
Exercise 1.1a
Check Your Learning Exercise (Text Version)
Matter consists of tiny particles that can combine in specific ratios to form substances with specific properties. Identify this statement as being most similar to a hypothesis, a law, or a theory. Explain your reasoning.
Check Your Answer[1]
Source: “Exercise 1.1a” is adapted from “Exercise 1.1-3b” in General Chemistry 1 & 2, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson, licensed under CC BY 4.0.
Exercise 1.1b
Check Your Learning Exercise (Text Version)
At a higher temperature, solids (such as salt or sugar) will dissolve better in water. Identify this statement as being most similar to a hypothesis, a law, or a theory. Explain your reasoning.
Check Your Answer[2]
Source: “Exercise 1.1b” is adapted from “Exercise 1.1-3c” from General Chemistry 1 & 2, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson, licensed under CC BY 4.0.
Exercise 1.1c
Check Your Learning Exercise (Text Version)
Identify the item in bold (also marked with an *) as a part of either the macroscopic domain, the microscopic domain, or the symbolic domain of chemistry. For those in the symbolic domain, indicate whether they are symbols for a macroscopic or a microscopic feature.
- A certain molecule contains one *H atom and one Cl atom.
- *Copper *wire has a density of about 8 g/cm3
- The bottle contains 15 grams of *Ni *powder.
- A *sulfur *molecule is composed of eight sulfur atoms.
Check Your Answer[3]
Source: “Exercise 1.1c” is adapted from “Exercise 1.1-5c” from General Chemistry 1 & 2, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson, licensed under CC BY 4.0.
Attribution & References
Study of the composition, properties, and interactions of matter
Tentative explanation of observations that acts as a guide for gathering and checking information
Statement that summarizes a vast number of experimental observations, and describes or predicts some aspect of the natural world.
Well-substantiated, comprehensive, testable explanation of a particular aspect of nature.
Path of discovery that leads from question and observation to law or hypothesis to theory, combined with experimental verification of the hypothesis and any necessary modification of the theory.
Realm of everyday things that are large enough to sense directly by human sight and touch.
Realm of things that are much too small to be sensed directly.
Specialized language used to represent components of the macroscopic and microscopic domains, such as chemical symbols, chemical formulas, chemical equations, graphs, drawings, and calculations.

