16.1 Acids and Bases
Learning Objectives
By the end of this section, you will be able to:
- Examine properties of acids and bases and provide examples of both
- Using a table for reference, differentiate between a strong or weak acid
- Using a table for reference, differentiate between a strong or weak base
- Define an Arrhenius acid and Arrhenius base
- Identify acids, bases, and conjugate acid-base pairs according to the Brønsted-Lowry definition
- Explain the Lewis model of acid-base chemistry
Many people enjoy drinking coffee. A cup first thing in the morning helps start the day. But keeping the coffee maker clean can be a problem. Lime deposits build up after a while and slow down the brewing process. The best cure for this is to put vinegar (dilute acetic acid) in the pot and run it through the brewing cycle. The vinegar dissolves the deposits and cleans the maker, which will speed up the brewing process back to its original rate. Just be sure to run water through the brewing process after the vinegar, or you will get some really horrible tasting coffee. Acids and bases are common substances found in many every day items, from fruit juices and soft drinks to soap. In this section, we'll explore the basic properties of acids and bases, and learn about the chemical nature of these important compounds.
Watch The Strengths and Weaknesses of Acids and Bases (3min 47s).
Video Source: Zaidan, George and Morton, Charles (TED-Ed) (2013, October 24). The strengths and weaknesses of acids and bases [Video]. YouTube.
Acids: Properties and Examples
Acids are very common in some of the foods that we eat. Citrus fruits such as oranges and lemons contain citric acid and ascorbic acid, which is better known as vitamin C. Carbonated sodas contain phosphoric acid. Vinegar contains acetic acid. Your own stomach utilizes hydrochloric acid to digest food. Acids are a distinct class of compounds because of the properties of their aqueous solutions as outlined below:
- Aqueous solutions of acids are electrolytes, meaning that they conduct electrical current. Some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. Other acids are weak electrolytes that exist primarily in a non-ionized form when dissolved in water.
- Acids have a sour taste. Lemons, vinegar, and sour candies all contain acids.
- Acids change the colour of certain acid-base indicators. Two common indicators are litmus and phenolphthalein. Blue litmus turns red in the presence of an acid, while phenolphthalein turns colourless.
- Acids react with bases to produce a salt compound and water. When equal moles of an acid and a base are combined, the acid is neutralized by the base. The products of this reaction are an ionic compound (a salt) and water.
- Acids react with active metals to yield hydrogen gas. Recall that an activity series is a list of metals in descending order of reactivity. Metals that are above hydrogen in the activity series will replace the hydrogen from an acid in a single-replacement reaction (Chapter 8.3), as shown below:
Zn(s) + H2SO4(aq) → ZnSO4(aq) + H2(g)
Table 16.1a provides a partial list of some common acids and their uses.
|
Compound Name and Chemical Formula
|
Common Name (if applicable) | Uses |
|---|---|---|
|
hydrochloric acid, HCl |
muriatic acid (used in pools) and stomach acid is HCl | Used in cleaning (refining) metals, in maintenance of swimming pools, and for household cleaning. |
|
sulfuric acid, H2SO4 |
none | Used in car batteries, and in the manufacture of fertilizers. |
|
nitric acid, HNO3 |
none | Used in the manufacture of fertilizers, explosives and in extraction of gold. |
|
acetic acid, HC2H3O2 |
vinegar | Main ingredient in vinegar. |
|
carbonic acid, H2CO3 |
responsible for the "fizz" in carbonated drinks | As an ingredient in carbonated drinks. |
|
citric acid, C6H8O7 |
none | Used in food and dietary supplements. Also added as an acidulant in creams, gels, liquids, and lotions. |
|
acetylsalicylic acid, C6H4(OCOCH3)CO2H |
aspirin | The active ingredient in aspirin. |
Later in this chapter, we will examine the chemistry behind what exactly makes an acid behave as an acid and what makes a base behave as a base. For now, take a look at the formulas given in the table 16.1a and take a guess as to what common feature characterizes an acid.

Bases: Properties and Examples
Perhaps you have eaten too much pizza and felt very uncomfortable hours later. This feeling is due to excess stomach acid being produced. The discomfort can be dealt with by taking an antacid. The base in the antacid will react with the HCl in the stomach and neutralize it, taking care of that unpleasant feeling. Bases have properties that mostly contrast with those of acids.
- Aqueous solutions of bases are also electrolytes. Bases can be either strong or weak, just as acids can.
- Bases often have a bitter taste and are found in foods less frequently than acids. Many bases, like soaps, are slippery to the touch.
- Bases also change the colour of indicators. Litmus turns blue in the presence of a base, while phenolphthalein turns pink as shown in Figure 16.1a
- Bases do not react with metals in the way that acids do but tend to react with fats and oils
- Bases react with acids to produce a salt and water.
Tasting chemicals and touching them are NOT good lab practices and should be avoided—in other words—don't do this at home. Bases are less common as foods, but they are nonetheless present in many household products. Many cleaners contain ammonia, a base. Sodium hydroxide is found in drain cleaner. Antacids, which combat excess stomach acid, are comprised of bases such as magnesium hydroxide or sodium hydrogen carbonate.
Various common bases and corresponding uses are given in Table 16.1b.
|
Compound Name and Chemical Formula
|
Common Name (if applicable)
|
Uses |
|---|---|---|
| sodium hydroxide, NaOH | (lye or caustic soda) | Used in the manufacture of soaps and detergents, and as the main ingredient in oven and drain cleaners. |
| potassium hydroxide, KOH | (lye or caustic potash) | Used in the production of liquid soaps and soft soaps. Used in alkaline batteries. |
| magnesium hydroxide, Mg(OH)2 | (milk of magnesia) | Used as an ingredient in laxatives, antacids, and deodorants. Also used in the neutralization of acidic wastewater. |
| calcium hydroxide, Ca(OH)2 | (slaked lime) | Used in the manufacture of cement and lime water. Also, added to neutralize acidic soil. |
| aluminum hydroxide, Al(OH)3 | none | Used in water purification and as an ingredient in antacids. |
| ammonia, NH3 | none | Used as a building block for the synthesis of many pharmaceutical products and in many commercial cleaning products. Used in the manufacture of fertilizers. |
Exercise 16.1a
Check Your Learning Exercise (Text Version)
Question 1 of Interactive:
For each of the following descriptions or properties, determine whether it is describing an acid or base:
- Typically have a bitter taste
- Solutions feel slippery
- Turns litmus paper red
- Turns litmus paper blue
- Typically react with metals, producing hydrogen bubbles (gas)
- Type of substance that aids in protein digestion in the stomach
- The active ingredient of aspirin is this type of substance
- Typically have a sour taste
- Baking soda is classified as this
- Citrus fruits are classified as this
- Antacids are classified as this
- Vinegar is classified as this
Question 2 of Interactive:
All of the follow are TRUE statements about bases EXCEPT
- often an ingredient in cleaning solutions and feel slippery
- when dissolved in water, have a bitter taste
- Are electrolytes – conduct electricity
- when metals are placed in a basic solution, they react, producing hydrogen bubbles
Question 3 of Interactive:
All of the follow are TRUE statements about ACIDS EXCEPT
- acids are found in citrus fruits and provide the bitter taste
- acids are found in citrus fruits and provide the sour taste
- turns litmus paper red
- Are electrolytes – conduct electricity
Question 4 of Interactive:
True or False? Both strong acids and bases can ionize in solution, which makes them electrolytes and can conduct an electrical current. However, only bases can dissolve metals, producing hydrogen gas.
Check Your Answer[1]
Source: "Exercise 16.1a" by Jackie MacDonald, licensed under CC BY-NC 4.0.
Strength of Acids and Bases
An acid or base's strength refers to its degree of ionization. We will explore the basics of acid/base strength below.
Strong and Weak Acids
Except for their names and formulas, so far we have treated all acids as equals, especially in a chemical reaction. However, acids can be very different in a very important way. Consider HCl(aq). When HCl is dissolved in water (H2O), it completely dissociates (100%) into H+(aq) and Cl−(aq) ions; all the HCl molecules become ions:
HCl → H+(aq) + Cl−(aq)
Any acid that dissociates 100% into ions is called a strong acid. If it does not dissociate 100%, it is a weak acid. Acetic Acid, HC2H3O2, is an example of a weak acid (it does not dissociate completely in aqueous solution, only about 5%):
HC2H3O2 → H+(aq) + C2H3O2-(aq)
Because this reaction does not go 100% to completion, it is more appropriate to write it as a reversible reaction:
HC2H3O2 ⇌ H+(aq) + C2H3O2-(aq)
As it turns out, there are very few strong acids, which are given in Table 16.1c. If an acid is not listed here, it is a weak acid. It may be 1% ionized or 99% ionized, but it is still classified as a weak acid.
| Acids | Bases |
|---|---|
| HCl | LiOH |
| HBr | NaOH |
| HI | KOH |
| HNO3 | RbOH |
| H2SO4 | CsOH |
| HClO3 | Mg(OH)2 |
| HClO4 | Ca(OH)2 |
| Sr(OH)2 | |
| Ba(OH)2 |
Strong and Weak Bases
The issue is similar with bases: a strong base is a base that is 100% ionized in solution. If it is less than 100% ionized in solution, it is a weak base. There are very few strong bases (Table 16.1c); any base not listed is a weak base. All strong bases are compounds containing hydroxide, OH–. Any other molecules classified as a base (using other mechanisms on defining bases), such as NH3 (which does not contain OH− ions as part of its formula), will be a weak base.
Molecular Definitions of Acids and Bases
Acids and bases have been known for a long time. When Robert Boyle characterized them in 1680, he noted that acids dissolve many substances, change the colour of certain natural dyes (for example, they change litmus from blue to red), and lose these characteristic properties after coming into contact with alkalis (bases). In the eighteenth century, it was recognized that acids have a sour taste, react with limestone to liberate a gaseous substance (now known to be CO2), and interact with alkalis to form neutral substances. In 1815, Humphry Davy contributed greatly to the development of the modern acid-base concept by demonstrating that hydrogen is the essential constituent of acids. Around that same time, Joseph Louis Gay-Lussac concluded that acids are substances that can neutralize bases and that these two classes of substances can be defined only in terms of each other. Three main classifications of acids and bases are predominantly used to explain acid base reactions:
- Arrhenius acids and bases: Acids yield protons when dissolved in solution, while Arrhenius bases yields hydroxide ions.
- Brønsted-Lowry acids and bases. Acids are proton donors, while Brønsted-Lowry bases are proton acceptors.
- Lewis acids and bases: Acids are electron acceptors, while Lewis bases are electron donors.
Arrhenius Acids/Bases
The significance of hydrogen was reemphasized in 1884 when Carl Axel Arrhenius defined an acid as a compound that dissolves in water to yield hydrogen cations (now recognized to be hydronium ions, H3O+) and a base as a compound that dissolves in water to yield hydroxide anions (OH-). In an earlier chapter on chemical reactions, we defined acids and bases as Arrhenius did:
- An acid (Arrhenius acid) as a compound that dissolves in water to produce H+ ions (in other words, yields hydronium ions, H3O+).
- A base (Arrhenius base) as a compound that dissolves in water to to yield hydroxide ions (OH−).
This definition is not wrong; it is simply limited. The theory does not explain the weak base ammonia (NH3), which in the presence of water, releases hydroxide ions into solution, but does not contain OH- itself. The Arrhenius definition of acid and base is also limited to aqueous solutions.
Brønsted-Lowry Acids/Bases
Later in 1923 the Danish chemist Johannes Brønsted and the English chemist Thomas Lowry proposed a more general definition of an acid or a base. Their definition centres on the proton, H+. A proton is what remains when a normal hydrogen atom, [latex]_1^1\text{H}[/latex], loses an electron. A compound that donates a proton to another compound is called a Brønsted-Lowry acid, and a compound that accepts a proton is called a Brønsted-Lowry base. An acid-base reaction is the transfer of a proton from a proton donor (acid) to a proton acceptor (base).
Acids may be compounds such as HCl or H2SO4, organic acids like acetic acid (CH3COOH) or ascorbic acid (vitamin C), or H2O. Anions (such as HSO4-, H2PO4- HS−, and HCO3- and cations (such as H3O+, NH4+, and [Al(H2O)5OH]2+) may also act as acids. Bases fall into the same three categories. Bases may be neutral molecules (such as H2O, NH3, and CH3NH2), anions (such as OH−, HS−, HCO3-, CO32-, F−, and PO43-), or cations (such as [Al(H2O)5OH]2+). The most familiar bases are ionic compounds such as NaOH and Ca(OH)2, which contain the hydroxide ion, OH−. The hydroxide ion in these compounds accepts a proton from acids to form water:
We call the product that remains after an acid donates a proton the conjugate base of the acid. This species is a base because it can accept a proton (to re-form the acid):
Example 16.1a
Write the conjugate base for each of the following acids: (Hint! Remember an acid will donate a proton)
- HClO4
- H3PO4
- CH3NH3+
Solution
- HClO4 is the acid; ClO4- is its conjugate base
- H3PO4 is the acid; H2PO4- is its conjugate base
- CH3NH3+ is the acid; CH3NH2 is its conjugate base
Source: "Example 16.1a" by Jackie MacDonald is licensed under CC BY-NC 4.0.
Exercise 16.1b
Write the conjugate base for each of the following acids:
- NH2–
- HBr
- HSO4-
Check Your Answer[2]
Source: "Exercise 16.1b" by Jackie MacDonald is licensed under CC BY-NC 4.0.
We call the product that results when a base accepts a proton the base’s conjugate acid. This species is an acid because it can give up a proton (and thus re-form the base):
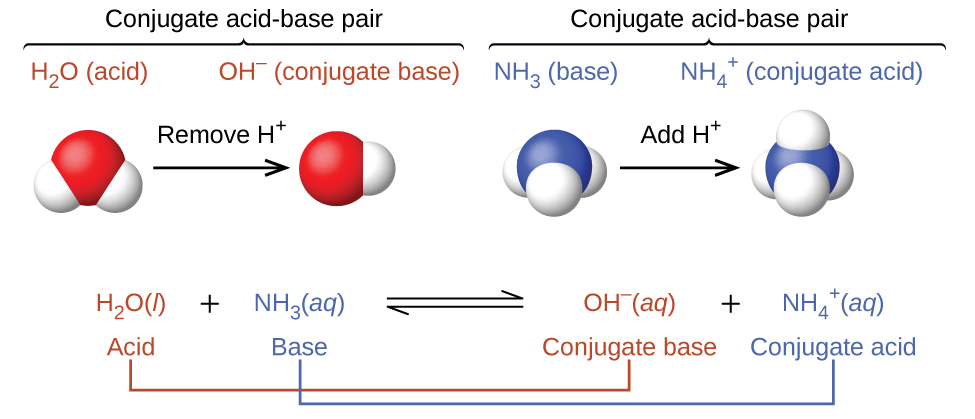
In these two sets of equations, the behaviours of acids as proton donors and bases as proton acceptors are represented in isolation. In reality, all acid-base reactions involve the transfer of protons between acids and bases. Interestingly, water can act as an acid or base depending on what gets dissolved in it. For example, consider the acid-base reaction that takes place when ammonia is dissolved in water (Figure 16.1b). A water molecule (functioning as an acid) transfers a proton to an ammonia molecule (functioning as a base), yielding the conjugate base of water, OH−, and the conjugate acid of ammonia, NH4+.
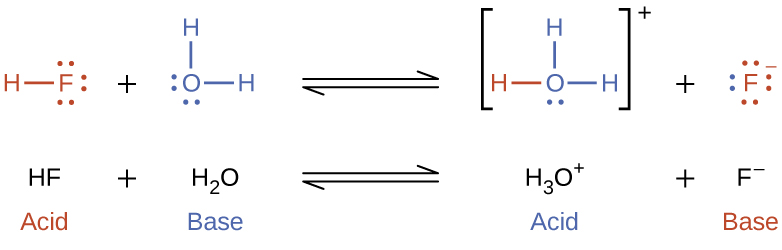
The reaction between a Brønsted-Lowry acid and water is called acid ionization. For example, when hydrogen fluoride dissolves in water and ionizes, protons are transferred from hydrogen fluoride molecules to water molecules, yielding hydronium ions and fluoride ions:
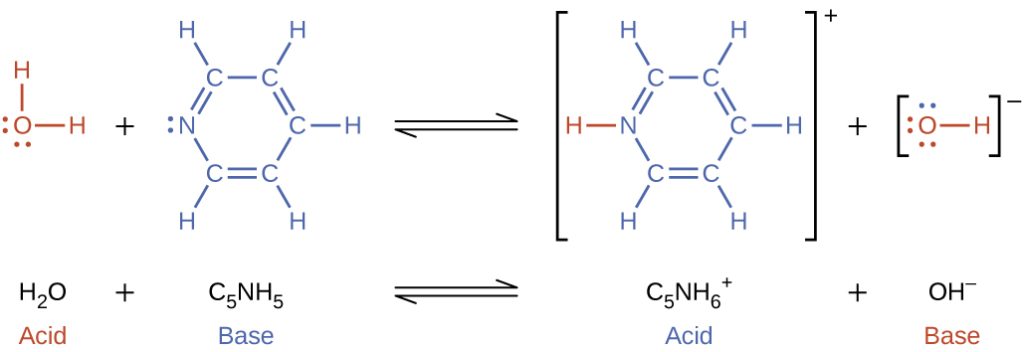
When we add a base to water, a base ionization reaction occurs in which protons are transferred from water molecules to base molecules. For example, adding pyridine to water yields hydroxide ions and pyridinium ions:
Notice that both these ionization reactions in Figure 16.1c and 16.1d are represented as equilibrium processes. The relative extent to which these acid and base ionization reactions proceed is an important topic that will be covered in the equilibrium chapter.
Watch Conjugate Acids & Bases | Acids, Bases & Alkali's | Chemistry | FuseSchool (3min 45sec).
Video Source: Fuse School - Global Education (2013, June 20). Conjugate acids & bases | Acids, bases & alkali's | Chemistry [Video]. YouTube.
Example 16.1b
Identify and label the Brønsted-Lowry acid, its conjugate base, the Brønsted-Lowry base, and its conjugate acid in each of the following equations:
- NO2- + H2O → HNO2 + OH−
- HBr + H2O → H3O+ + Br−
Solution
- H2O is the Brønsted-Lowry acid and OH− is its conjugate base; NO2- is the Brønsted-Lowry base and HNO2 is its conjugate acid.
- HBr is the Brønsted-Lowry acid and Br− is its conjugate base; H2O is the Brønsted-Lowry base and H3O+ is its conjugate acid.
Exercise 16.1c
Identify and label the Brønsted-Lowry acid, its conjugate base, the Brønsted-Lowry base, and its conjugate acid in each of the following equations:
- HS− + H2O → H2S + OH−
- H2PO4- + OH− → HPO42- + H2O
- H2PO4- + HCl → H3PO4 + Cl−
- [Fe(H2O)5(OH)]2+ + [Al(H2O)6]3+ → [Fe(H2O)6]3+ + [Al(H2O)5(OH)]2+
Check Your Answer[3]
Exercise 16.1d
Check Your Learning Exercise (Text Version)
- Correctly match each term with its correct definition:
TERMS: (i) Brønsted-Lowry acid; (ii) Brønsted-Lowry base
DEFINITIONS: (1) A compound that donates a proton to another compound; (2) a compound that accepts a proton - What is the conjugate acid in the following equation? PO43- + HNO3 → NO3- + HPO42-
- What is the conjugate base in the following equation? HCO3- + HCl → H2CO3 + Cl-
- Which of the following is the correct Brønsted-Lowry acid, conjugate base pair for the following reaction: CH3OH + H− → CH3O− + H2
- Brønsted-Lowry acid CH3O−, conjugate base CH3OH
- Brønsted-Lowry acid CH3OH, conjugate base CH3O−
- Brønsted-Lowry acid H− , conjugate base H2
- Brønsted-Lowry acid CH3OH, conjugate base H−
Check Your Answer[4]
Source: "Exercise 16.1d" by Jackie MacDonald, licensed under CC BY-NC 4.0.
Lewis Acids and Bases
In 1923, G. N. Lewis proposed a generalized definition of acid-base behaviour in which acids and bases are identified by their ability to accept or to donate a pair of electrons and form a coordinate covalent bond.
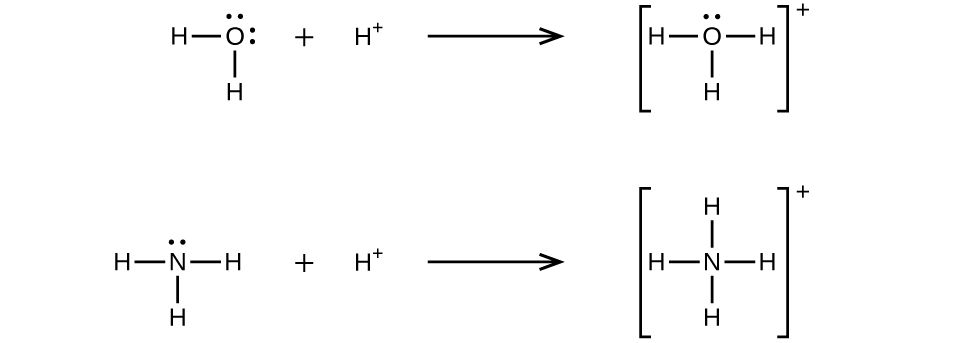
A coordinate covalent bond (or dative bond) occurs when one of the atoms in the bond provides both bonding electrons. For example, a coordinate covalent bond occurs when a water molecule combines with a hydrogen ion to form a hydronium ion. A coordinate covalent bond also results when an ammonia molecule combines with a hydrogen ion to form an ammonium ion. Both of these equations are shown in Figure 16.1e.
A Lewis acid is any species (molecule or ion) that can accept a pair of electrons, and a Lewis base is any species (molecule or ion) that can donate a pair of electrons.
A Lewis acid-base reaction occurs when a base donates a pair of electrons to an acid. A Lewis acid-base adduct, a compound that contains a coordinate covalent bond between the Lewis acid and the Lewis base, is formed. The following equations illustrate the general application of the Lewis concept.
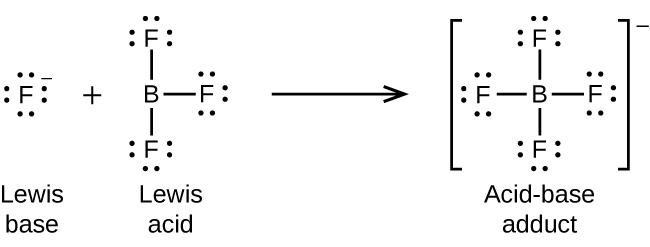
The boron atom in boron trifluoride, BF3, has only six electrons in its valence shell. Being short of the preferred octet, BF3 is a very good Lewis acid and reacts with many Lewis bases; a fluoride ion is the Lewis base in this reaction, donating one of its lone pairs as demonstrated in Figure 16.1f:
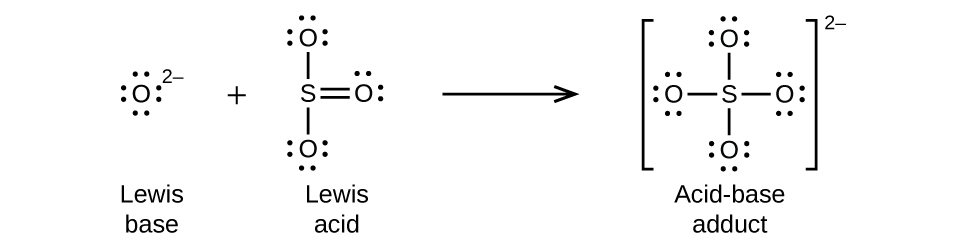
Nonmetal oxides act as Lewis acids and react with oxide ions, Lewis bases, to form oxyanions as shown in Figure 16.1g.
Many Lewis acid-base reactions are displacement reactions in which one Lewis base displaces another Lewis base from an acid-base adduct, or in which one Lewis acid displaces another Lewis acid (Figure 16.1h):
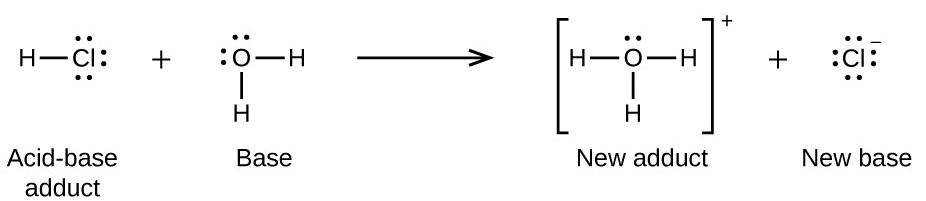
The displacement reaction in Figure 16.1h shows how the reaction of a Brønsted-Lowry acid with a base fits into the Lewis concept. A Brønsted-Lowry acid such as HCl is an acid-base adduct according to the Lewis concept, and proton transfer occurs because a more stable acid-base adduct is formed. Thus, although the definitions of acids and bases in the two theories are quite different, the theories overlap considerably.
Amphiprotic / Amphoteric Species
As illustrated in the previous paragraphs water can behave as an acid or a base. Like water, many molecules and ions may either gain or lose a proton under the appropriate conditions. Such species are said to be amphiprotic. Another term used to describe such species is amphoteric, which is a more general term for a species that may act either as an acid or a base by any definition (not just the Brønsted-Lowry one). Consider for example the bicarbonate ion, which may either donate or accept a proton as shown here:
Example 16.1c
Representing the Acid-Base Behaviour of an Amphoteric Substance
Write separate equations representing the reaction of HSO3-.
- as an acid with OH−
- as a base with HI
Solution
- [latex]\text{HSO}_3^{\;\;-}(aq)\;+\;\text{OH}^{-}(aq)\;{\leftrightharpoons}\;\text{SO}_3^{\;\;2-}(aq)\;+\;\text{H}_2\text{O}(l)[/latex]
- [latex]\text{HSO}_3^{\;\;-}(aq)\;+\;\text{HI}(aq)\;{\leftrightharpoons}\;\text{H}_2\text{SO}_3(aq)\;+\;\text{I}^{-}(aq)[/latex]
Exercise 16.1e
Write separate equations representing the reaction of H2PO4.
- as a base with HBr
- as an acid with OH−
Check Your Answer[5]
Links to Interactive Learning Tools
Explore Acid-Base Properties from the Physics Classroom.
Explore Bronsted-Lowry Acid and Base Model from the Physics Classroom.
Attribution & References
Except where otherwise noted, this section is adapted by Jackie MacDonald from "14.2: Acids- Properties and Examples", "14.3: Bases- Properties and Examples", and "14.7: Strong and Weak Acids and Bases" In Map: Introductory Chemistry (Tro) by Marisa Alviar-Agnew & Henry Agnew, Shared under CK-12 license. / Content streamlined and remixed for student comprehension.
- "Molecular Definitions of Acids and Bases" and "Amphiprotic / Amphoteric Species" sections are adapted from "14.1 Brønsted-Lowry Acids and Bases" and "15.2 Lewis Acids and Bases" In General Chemistry 1 & 2 by Rice University, licensed under CC BY 4.0 International License, Except where otherwise noted. / Wording modifications by Jackie MacDonald.
-
Question 1:
- base;
- base;
- acid;
- base;
- acid;
- acid;
- acid;
- acid;
- base;
- acid;
- base;
- acid;
-
- when NH2– is the acid; NH2- is its conjugate base;
- when HBr the acid; Br- is its conjugate base;
- When HSO4- is the acid; SO42- is the base.
-
- H2O is the Brønsted-Lowry acid and OH− is its conjugate base; HS− is the Brønsted-Lowry base and H2S is its conjugate acid;
- H2PO4- is the Brønsted-Lowry acid and HPO42- is its conjugate base; OH− is the Brønsted-Lowry base and H2O is its conjugate acid;
- HCl is the Brønsted-Lowry acid and Cl− is its conjugate base; H2PO4- is the Brønsted-Lowry base and H3PO4 is its conjugate acid;
- [Al(H2O)6]3+ is the Brønsted-Lowry acid and [Al(H2O)5(OH)]2+ is its conjugate base; [Fe(H2O)5(OH)]2+ is the Brønsted-Lowry base and [Fe(H2O)6]3+ is its conjugate acid.
-
- (i) Brønsted-Lowry acid = (1) A compound that donates a proton to another compound; (ii) Brønsted-Lowry base = (2) a compound that accepts a proton
- HPO42-
- Cl-
- 2. Brønsted-Lowry acid CH3OH, conjugate base CH3O−
-
- [latex]\text{H}_2\text{PO}_4^{\;\;-}(aq)\;+\;\text{HBr}(aq)\;{\leftrightharpoons}\;\text{H}_3\text{PO}_4(aq)\;+\;\text{Br}^{-}(aq)[/latex];
- [latex]\text{H}_2\text{PO}_4^{\;\;-}(aq)\;+\;\text{OH}^{-}(aq)\;{\leftrightharpoons}\;\text{HPO}_4^{\;\;2-}(aq)\;+\;\text{H}_2\text{O}(l)[/latex]
acid that reacts completely when dissolved in water to yield hydronium ions
acid that reacts only to a slight extent when dissolved in water to yield hydronium ions
base that reacts completely when dissolved in water to yield hydroxide ions
base that reacts only to a slight extent when dissolved in water to yield hydroxide ions
An acid as a compound that dissolves in water to produce H+ ions (hydronium ions, H3O+)
a compound that dissolves in water to to yield hydroxide ions (OH−)
A compound that donates a proton to another compound
a compound that accepts a proton
the product that remains after an acid donates a proton
the product that results when a base accepts a proton
(also, dative bond) bond formed when one atom provides both electrons in a shared pair
any species that can accept a pair of electrons and form a coordinate covalent bond
any species that can donate a pair of electrons and form a coordinate covalent bond
compound or ion that contains a coordinate covalent bond between a Lewis acid and a Lewis base
Able to both donate and accept a proton, and thus able to react both as an acid and a base
species that can act as either an acid or a base

