Chapter 15 – Review
15.1 Salts
- Explain how a salt is formed.
Check answers: [1] - What salt will form when you combine hydrobromic acid and lithium hydroxide?
Check answers: [2] - What salt will form when you combine sulfuric acid, H2SO4, and sodium hydroxide?
Check answers: [3]
15.2 Electrolytes
- Differentiate between strong and weak electrolytes.
Check Answers: [4] - Explain why the ions Na+ and Cl− are strongly solvated in water but not in hexane, a solvent composed of nonpolar molecules. Check Answer: [5]
- Explain why solutions of HBr in benzene (a nonpolar solvent) are nonconductive, while solutions in water (a polar solvent) are conductive.
- Consider the solutions presented:
- Which of the following sketches, shown in the figure below, best represents the ions in a solution of Fe(NO3)3(aq)?
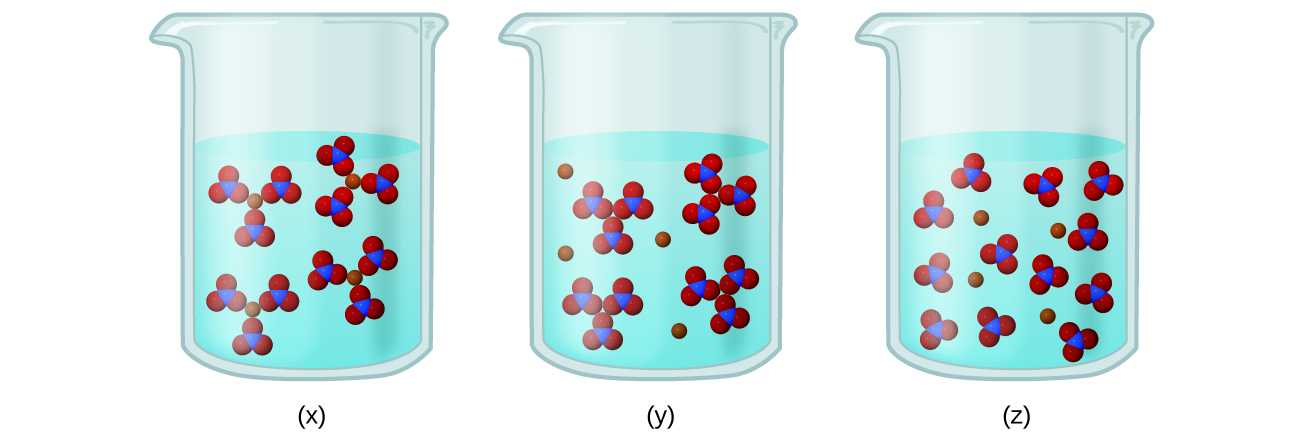
-
Write a balanced chemical equation showing the products of the dissolution of Fe(NO3)3.
Check Answer: [6]
- Which of the following sketches, shown in the figure below, best represents the ions in a solution of Fe(NO3)3(aq)?
- Compare the processes that occur when methanol (CH3OH), hydrogen chloride (HCl), and sodium hydroxide (NaOH) dissolve in water. Write equations and prepare sketches showing the form in which each of these compounds is present in its respective solution.
- What is the expected electrical conductivity of the following solutions?
- NaOH(aq)
- HCl(aq)
- C6H12O6(aq) (glucose)
- NH3(l)
Check Answer: [7]
- Why are most solid ionic compounds electrically nonconductive, whereas aqueous solutions of ionic compounds are good conductors? Would you expect a liquid (molten) ionic compound to be electrically conductive or nonconductive? Explain.
- Indicate the most important type of intermolecular attraction responsible for solvation in each of the following solutions:
- the solutions in Figure 14.2c
- methanol, CH3OH, dissolved in ethanol, C2H5OH
- methane, CH4, dissolved in benzene, C6H6
- the polar halocarbon CF2Cl2 dissolved in the polar halocarbon CF2ClCFCl2
- O2(l) in N2(l)
Check Answer: [8]
15.3 Precipitation Reactions
- What are the general characteristics that help you recognize double replacement reactions?
Check Answers: [9] - What are the general characteristics that help you recognize a precipitation reaction?
Check Answers: [10] - Assuming that the following is a precipitation reaction, determine the products (and identify their phases, aq or s) and write the balanced chemical equation.
Zn(NO3)2 + NaOH → ?
Check Answers: [11] - Assuming that the following is a precipitation reaction, determine the products (and identify their phases, aq or s) and write the balanced chemical equation.
MgCl2 + NaOH →
Check Answers: [12] - Use the solubility table to predict if the following double replacement reaction will occur and, if so, write a balanced chemical equation. Pb(NO3)2 + KBr → ?
Check Answers: [13] - Use the solubility table to predict if the following double replacement reaction will occur and, if so, write a balanced chemical equation.
KCl + Na2CO3 → ?
Check Answers:[14] - Which solution could be used to precipitate the barium ion, Ba2+, in a water sample: sodium chloride, sodium hydroxide, or sodium sulfate? What is the formula for the expected precipitate?
Check Answers: [15]
15.4 Net Ionic Equations
- From the balanced molecular equations, write the complete ionic and net ionic equations for the following:
(a) K2C2O4(aq) + Ba(OH)2 → 2KOH(aq) + BaC2O2(s)
(b) Pb(NO3)2(aq) + H2SO4(aq) → PbSO4(s) +2HNO3(aq)
(c) CaCO3(s) + H2SO4(aq) → CaSO4(s) + CO2(g) + H2O(l)
Check Answers: [16] - Predict the result of mixing reasonably concentrated solutions of the following ionic compounds. If precipitation is expected, write a balanced net ionic equation for the reaction.
(a) potassium sulfate and barium nitrate
(b) lithium chloride and silver acetate
(c) lead nitrate and ammonium carbonate
Check Answers: [17] - From the balanced molecular equation, write the net ionic equations for the following reaction:
2HCl(aq) + Ba(OH)2(aq) → BaCl2(aq) + H2O(l)
Check Answers: [18] - From the balanced molecular equation, write the net ionic equations for the following reaction:
2AgNO3(aq) + Cu(s) → Cu(NO3)2(aq) + 2Ag(s)
Check Answers: [19]
Attribution & References
Except where otherwise noted, this page is adapted by Jackie MacDonald from:
- 15.1 Salts & 15.2 Electrolytes, 15.3 Precipitation Reactions (#2, 4, 6), 15.4 Net Ionic Equations (#3 & #4) – Questions created by Jackie MacDonald, licensed under CC BY 4.0
- 15.3 Precipitation Reactions – Questions 1, 3, 5, 7 – adapted from “4.2 Classifying Chemical Reactions“In CHEM 1114 – Introduction to Chemistry by Shirley Wacowich-Sgarbi and Langara Chemistry Department is licensed under CC BY-NC-SA 4.0.
- 15.4 Net Ionic Equations, questions 1 & 2 are adapted from “4.1 Writing and Balancing Chemical Equations” and “4.2 Classifying Chemical Reactions“In CHEM 1114 – Introduction to Chemistry by Shirley Wacowich-Sgarbi and Langara Chemistry Department is licensed under CC BY-NC-SA 4.0.Adaptations and additions were made to content from these sections for student comprehension.
- A salt is formed in a neutralization reaction of an acid and a base. Acids and bases always contain either a metal cation or a cation derived from ammonium (NH4+) and a nonmetal anion, the two can combine to form a salt. ↵
- the anion from the HBr is Br-. The cation from LiOH is Li+. The salt formed is LiBr, lithium bromide. ↵
- the anion from the H2SO4 is SO42-. The cation from NaOH is Na+. The salt formed is Na2SO4, sodium sulfate. ↵
- A strong electrolyte is a salt that fully ionizes (100%) in the aqueous solution and are great conductors of electricity. Whereas, with a weak electrolyte only a relatively small fraction of the dissolved substance undergoes the ion-producing process and therefore is a poor conductor of electricity. ↵
- Crystals of NaCl dissolve in water, a polar liquid with a very large dipole moment, and the individual ions become strongly solvated. Hexane is a nonpolar liquid with a dipole moment of zero and, therefore, does not significantly interact with the ions of the NaCl crystals. ↵
- (a) Fe(NO3)3 is a strong electrolyte, thus it should completely dissociate into Fe3+ and ([latex]\text{NO}_3^{\;\;-}[/latex]) ions. Therefore, (z) best represents the solution. (b) [latex]\text{Fe(NO}_3)_3(s)\;{\longrightarrow}\;\text{Fe}^{3+}(aq)\;+\;3\text{NO}_3^{\;\;-}(aq)[/latex] ↵
- (a) high conductivity (solute is an ionic compound that will dissociate when dissolved); (b) high conductivity (solute is a strong acid and will ionize completely when dissolved); (c) nonconductive (solute is a covalent compound, neither acid nor base, unreactive towards water); (d) low conductivity (solute is a weak base and will partially ionize when dissolved) ↵
- (a) ion-dipole; (b) hydrogen bonds; (c) dispersion forces; (d) dipole-dipole attractions; (e) dispersion forces ↵
- A double replacement reaction occurs when parts of two ionic compounds are exchanged, making two new compounds. A characteristic of a double-replacement equation is that there are two compounds as reactants and two different compounds as products. ↵
- In a precipitation reactions, the reactants will be dissolved substances in aqueous solutions and will react to form one (or more) solid products. Many reactions of this type involve the exchange of ions between ionic compounds in aqueous solution and a type of double displacement/replacement reaction. ↵
- Zn(NO3)2(aq) + 2NaOH(aq) → 2NaNO3(aq) + Zn(OH)2(s) ↵
- MgCl2(aq) + 2NaOH(aq) → 2NaCl(aq) + Mg(OH)2(s) ↵
- The reaction will occur since a precipitate will form. Pb(NO3)2(aq) + 2KBr(aq) → 2KNO3(aq) + PbBr2(s) ↵
- No reaction occurs; both products are soluble in water. 2KCl(aq) + Na2CO3(aq) → 2NaCl(aq) + K2CO3(aq) ↵
- sodium sulfate, BaSO4 ↵
- (a) complete ionic: 2K+(aq) + C2O42−(aq) + Ba2+(aq) + 2OH−(aq) → 2K+(aq) + 2OH−(aq) + BaC2O4(s) net ionic: Ba2+(aq) + C2O42−(aq) → BaC2O4(s) (b) complete ionic: Pb2+(aq) + 2NO3−(aq) + 2H+(aq) + SO42−(aq) → PbSO4(s) + 2H+(aq) + 2NO3−(aq) net ionic: Pb2+(aq) + SO42−(aq) → PbSO4(s) (c) complete ionic: CaCO3(s) + 2H+(aq) + SO42−(aq) → CaSO4(s) + CO2(g) + H2O(l) net ionic: CaCO3(s) + 2H+(aq) + SO42−(aq) → CaSO4(s) + CO2(g) + H2O(l) ↵
- (a) The two possible products for this combination are KNO3 and BaSO4. The solubility guidelines indicate BaSO4 is insoluble, and so a precipitation reaction is expected. The net ionic equation for this reaction isBa2+(aq) + SO42−(aq) → BaSO4(s) (b) The two possible products for this combination are LiC2H3O2 and AgCl. The solubility guidelines indicate AgCl is insoluble, and so a precipitation reaction is expected. The net ionic equation for this reaction isAg+(aq) + Cl−(aq) → AgCl(s) (c) The two possible products for this combination are PbCO3 and NH4NO3. The solubility guidelines indicate PbCO3 is insoluble, and so a precipitation reaction is expected. The net ionic equation for this reaction isPb2+(aq) + CO32−(aq) → PbCO3(s) ↵
- H+(aq) + OH-(aq) → H2O(l) ↵
- 2Ag+(aq) + Cu(s) → Cu2+(aq) + 2Ag(s) ↵

