10.5 Atomic Structures of the First 20 Elements
Learning Objectives
By the end of this section, you will be able to:
- Derive the predicted ground-state electron configurations of atoms
- Identify and explain exceptions to predicted electron configurations for atoms and ions
- Relate electron configurations to element classifications in the periodic table
Having introduced the basics of atomic structure and quantum mechanics, we can use our understanding of quantum numbers to determine how atomic orbitals relate to one another. This allows us to determine which orbitals are occupied by electrons in each atom. The specific arrangement of electrons in orbitals of an atom determines many of the chemical properties of that atom.
Orbital Energies and Atomic Structure
The energy of atomic orbitals increases as the principal quantum number, n, increases. In any atom with two or more electrons, the repulsion between the electrons makes energies of subshells with different values of l differ so that the energy of the orbitals increases within a shell in the order s < p < d < f. Figure 10.5a depicts how these two trends in increasing energy relate. The 1s orbital at the bottom of the diagram is the orbital with electrons of lowest energy. The energy increases as we move up to the 2s and then 2p, 3s, and 3p orbitals, showing that the increasing n value has more influence on energy than the increasing l value for small atoms. However, this pattern does not hold for larger atoms with more electrons The 3d orbital is higher in energy than the 4s orbital. Such overlaps continue to occur frequently as we move up the chart.
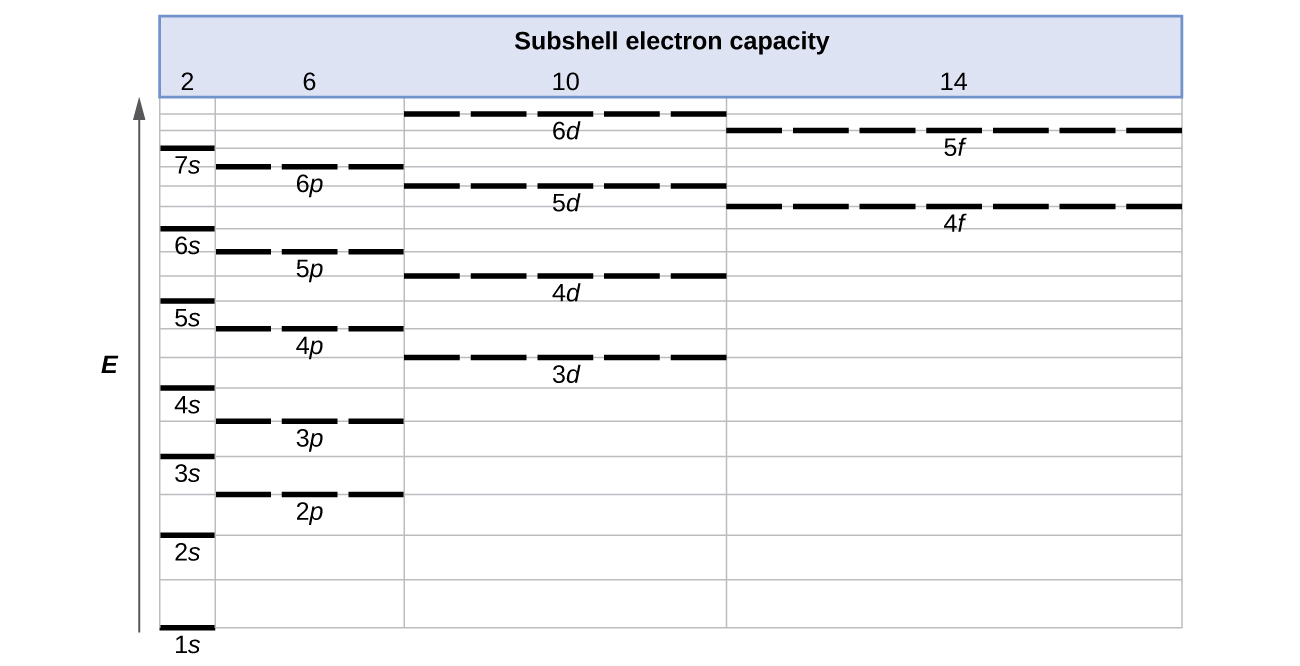
Electrons in successive atoms on the periodic table tend to fill low-energy orbitals first. Thus, many students find it confusing that, for example, the 5p orbitals fill immediately after the 4d, and immediately before the 6s. The filling order is based on observed experimental results, and has been confirmed by theoretical calculations. As the principal quantum number, n, increases, the size of the orbital increases and the electrons spend more time farther from the nucleus. Thus, the attraction to the nucleus is weaker and the energy associated with the orbital is higher (less stabilized), consistent with Coulomb's Law. But this is not the only effect we have to take into account. Within each shell, as the value of l increases, the electrons are less penetrating (meaning there is less electron density found close to the nucleus), in the order s > p > d > f. Electrons that are closer to the nucleus slightly repel electrons that are farther out, offsetting the more dominant electron–nucleus attractions slightly (recall that all electrons have −1 charges, but nuclei have +Z charges). This phenomenon is called shielding. Electrons in orbitals that experience more shielding are less stabilized and thus higher in energy. For small orbitals (1s through 3p), the increase in energy due to n is more significant than the increase due to l; however, for larger orbitals the two trends are comparable and cannot be simply predicted. We will discuss methods for remembering the observed order.
The arrangement of electrons in the orbitals of an atom is commonly represented using two methods: orbital diagrams and electron configurations of an atom. Both methods will be introduced in this section. It is important to apply the electron capacity rules for each type of subshell (l):
- electron capacity for subshell s is 2
- electron capacity for subshell p is 6
- electron capacity for subshell d is 10
- electron capacity for subshell f is 14
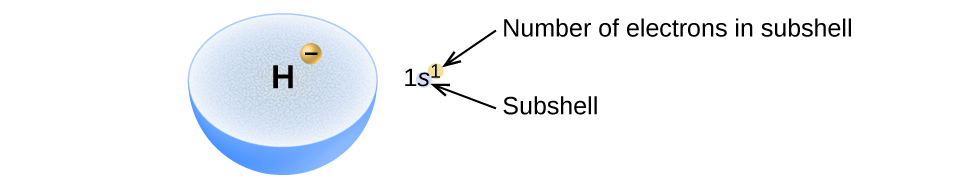
We write an electron configuration with a symbol that contains three pieces of information (Figure 10.5b):
- The number of the principal energy level (shell), n,
- The letter that designates the orbital type (the subshell, l), and
- A superscript number that designates the number of electrons in that particular subshell.
For example, the notation 2p4 (read "two–p–four") indicates four electrons in a p subshell (l = 1) with a principal quantum number (n) of 2. The notation 3d8 (read "three–d–eight") indicates eight electrons in the d subshell (i.e., l = 2) of the principal shell for which n = 3.
The Aufbau Principle
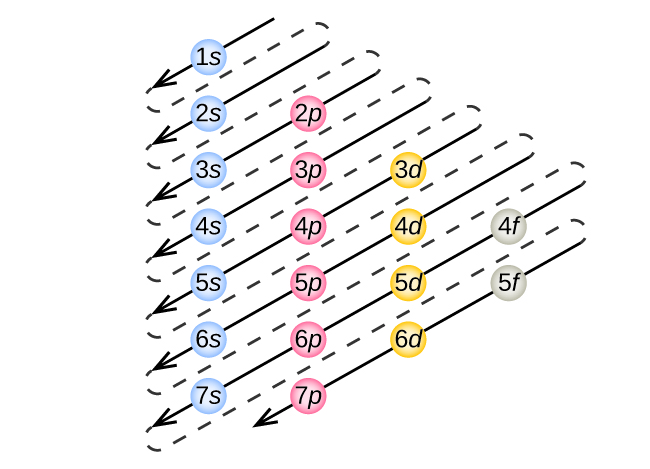
To determine the electron configuration (electron filling order) for any particular atom, we can “build” the structures in the order of atomic numbers. Beginning with hydrogen, and continuing across the periods of the periodic table, we add one proton at a time to the nucleus and one electron to the proper subshell until we have described the electron configurations of all the elements. This procedure is called the Aufbau principle, from the German word Aufbau (“to build up”). Each added electron occupies the subshell of lowest energy available (in the order shown in Figure 10.5a), subject to the limitations imposed by the allowed quantum numbers according to the Pauli exclusion principle. Electrons enter higher-energy subshells only after lower-energy subshells have been filled to capacity. Figure 10.5c illustrates the traditional way to remember the filling order for atomic orbitals. It is a helpful schematic to use when writing electron configurations or drawing orbital diagrams.
For an introduction on how to use the Orbital Filling Diagram and Aufbau's principle to write electron configurations watch Using the Electron Configuration Chart (3min 32s)
Video Source: Breslyn, W. (2013, November 12). Using the electron configuration chart [Video]. YouTube.
Electron Configuration Arrangement using the Periodic Table
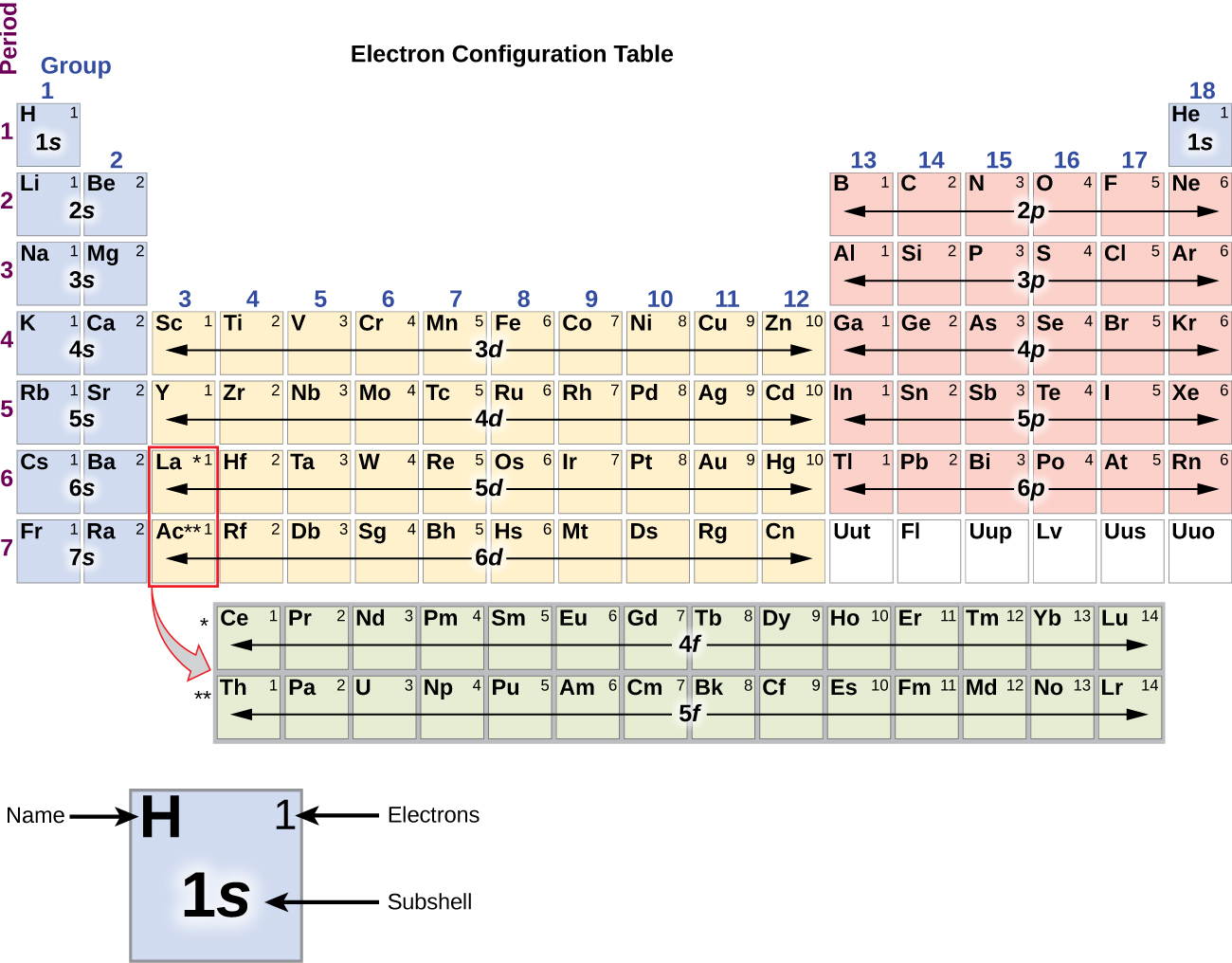
Since the arrangement of the periodic table is based on the electron configurations, the periodic table can be converted to an electron configuration table to map out electron filling order. Figure 10.5d illustrates this method for determining the electron configuration. The filling order simply begins at hydrogen and includes each subshell as you proceed in increasing Z order. For example, after filling the 3p block up to Argon (Ar), we see the next orbital to be filled with electrons will be 4s (for potassium (K) and calcium (Ca)), followed by the 3d orbitals.
When filling electrons to create electron configurations and orbital diagrams, remember the number of electrons increases by one as the atomic number increases by one.
For an introduction on how to use the periodic table to write electron configurations, watch Writing Electron Configurations Using Only the Periodic Table (4min 51s).
Video Source: Breslyn, W. (2013, November 13). Writing electron configurations using only the periodic table [Video]. YouTube.
Writing Electron Configuration and Orbital Diagrams of Elements
We will now construct the ground-state electron configuration and orbital diagram for a selection of atoms in the first and second periods of the periodic table. You can use the orbital filling diagram or your periodic table as tools to determine correct filling order. Orbital diagrams are pictorial representations of the electron configuration, showing the individual orbitals and the pairing arrangement of electrons. Boxes are drawn to represent each orbital (which can only contain zero, one, or two electrons). The orbitals' n value and l value are written under the box. Small arrows are used to indicate electrons. If two electrons share the same orbital, the first is drawn pointing in the up direction and the other in the down direction; this illustrates that the two electrons have opposite spins.
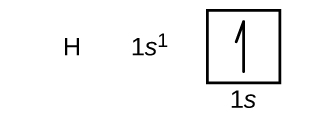
We start with a single hydrogen atom (atomic number 1), which consists of one proton and one electron. Referring to Figure 10.5c or Figure 10.5d, we would expect to find the electron in the 1s orbital. By convention, the [latex]m_s = + \frac{1}{2}[/latex] value is usually filled first. The symbol for hydrogen, its electron configuration, and its orbital diagram, respectively, are:
Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron (n = 1, l = 0, ml = 0, [latex]m_s = + \frac{1}{2}[/latex]). The second electron also goes into the 1s orbital and fills that orbital. The second electron has the same n, l, and ml quantum numbers, but must have the opposite spin quantum number, [latex]m_s = - \frac{1}{2}[/latex]. This is in accordance with the Pauli exclusion principle: No two electrons in the same atom can have the same set of four quantum numbers. For orbital diagrams, this means two arrows go in each box (representing two electrons in each orbital) and the arrows must point in opposite directions (representing paired spins). The electron configuration and orbital diagram of helium are:
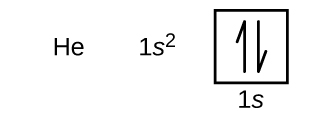
The n = 1 shell is completely filled in a helium atom.
The next atom is the alkali metal lithium, with an atomic number of 3, which means it has three electrons to fill. The first two electrons in lithium fill the 1s orbital and have the same sets of four quantum numbers as the two electrons in helium. The remaining electron must occupy the orbital of next lowest energy, the 2s orbital (Figure 10.5c or Figure 10.5d). Thus, the electron configuration and orbital diagram of lithium are:
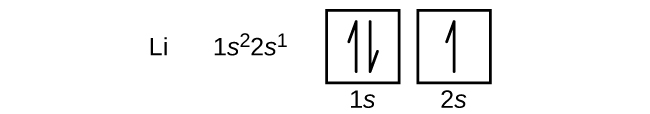
An atom of the alkaline earth metal beryllium, with an atomic number of 4, contains four protons in the nucleus and four electrons surrounding the nucleus. The fourth electron fills the remaining space in the 2s orbital.
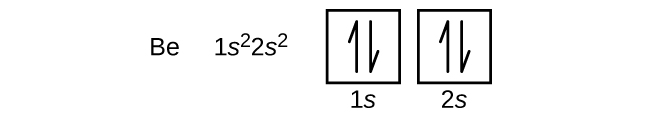
An atom of boron (atomic number 5) contains five electrons. The n = 1 shell is filled with two electrons and three electrons will occupy the n = 2 shell. Because any s subshell can contain only two electrons, the fifth electron must occupy the next energy level, which will be a 2p orbital. There are three degenerate 2p orbitals (ml = −1, 0, +1) and the electron can occupy any one of these p orbitals. When drawing orbital diagrams, we include empty boxes to depict any empty orbitals in the same subshell that we are filling.
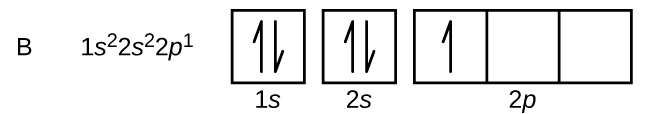
Carbon (atomic number 6) has six electrons. Four of them fill the 1s and 2s orbitals. The remaining two electrons occupy the 2p subshell. We now have a choice of filling one of the 2p orbitals and pairing the electrons or of leaving the electrons unpaired in two different, but degenerate, p orbitals. The orbitals are filled as described by Hund’s rule: the lowest-energy configuration for an atom with electrons within a set of degenerate orbitals is that having the maximum number of unpaired electrons. Thus, the two electrons in the carbon 2p orbitals have identical n, l, and ms quantum numbers and differ in their ml quantum number (in accord with the Pauli exclusion principle). The electron configuration and orbital diagram for carbon are:
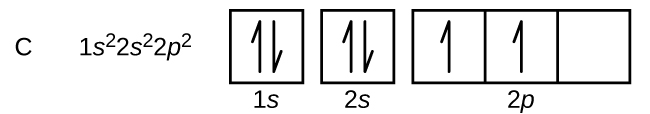
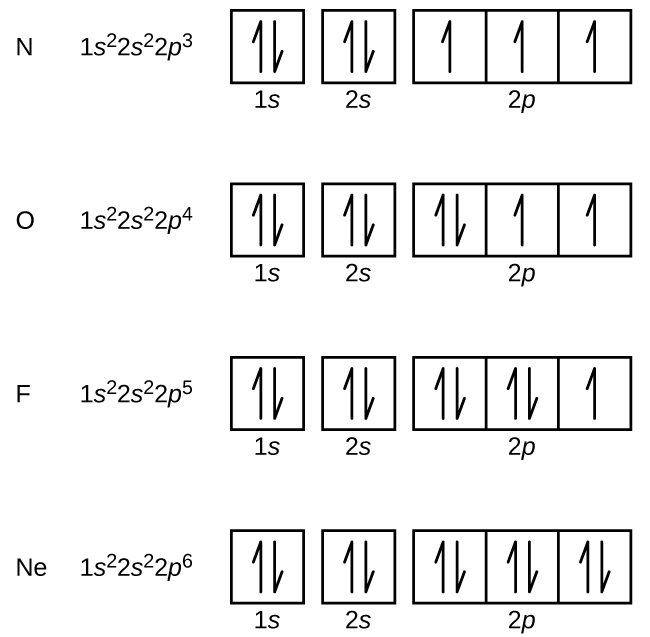
Nitrogen (atomic number 7) fills the 1s and 2s subshells and has one electron in each of the three 2p orbitals, in accordance with Hund’s rule (electrons fill each orbital first, then double up). These three electrons have unpaired spins. Oxygen (atomic number 8) has a pair of electrons in any one of the 2p orbitals (the electrons have opposite spins) and a single electron in each of the other two. Fluorine (atomic number 9) has only one 2p orbital containing an unpaired electron. All of the electrons in the noble gas neon (atomic number 10) are paired, and all of the orbitals in the n = 1 and the n = 2 shells are filled. The electron configurations and orbital diagrams of these four elements are:
![This figure includes the element symbol N a, followed by the electron configuration for the element. The first part of the electron configuration, 1 s superscript 2 2 s superscript 2 2 p superscript 6, is shaded in purple and is labeled, “core electrons.” The last portion, 3 s superscript 1, is shaded orange and is labeled, “valence electron.” To the right of this configuration is the word “Abbreviation” followed by [ N e ] 3 s superscript 1.](https://ecampusontario.pressbooks.pub/app/uploads/sites/3164/2023/03/CNX_Chem_06_04_Valence.jpg)
Similarly, the abbreviated configuration of lithium can be represented as [He]2s1, where [He] represents the configuration of the helium atom, which is identical to that of the filled inner shell of lithium. Writing the configurations in this way emphasizes the similarity of the configurations of lithium and sodium. Both atoms, which are in the alkali metal family, have only one electron in a valence s subshell outside a filled set of inner shells.
The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [Ne]3s2 configuration, is analogous to its family member beryllium, [He]2s2. Both atoms have a filled s subshell outside their filled inner shells. Aluminum (atomic number 13), with 13 electrons and the electron configuration [Ne]3s23p1, is analogous to its family member boron, [He]2s22p1.
The electron configurations of silicon (14 electrons), phosphorus (15 electrons), sulfur (16 electrons), chlorine (17 electrons), and argon (18 electrons) are analogous in the electron configurations of their outer shells to their corresponding family members carbon, nitrogen, oxygen, fluorine, and neon, respectively, except that the principal quantum number of the outer shell of the heavier elements has increased by one to n = 3. Figure 10.5m shows the lowest energy, or ground-state, electron configuration for these elements as well as that for atoms of each of the known elements.
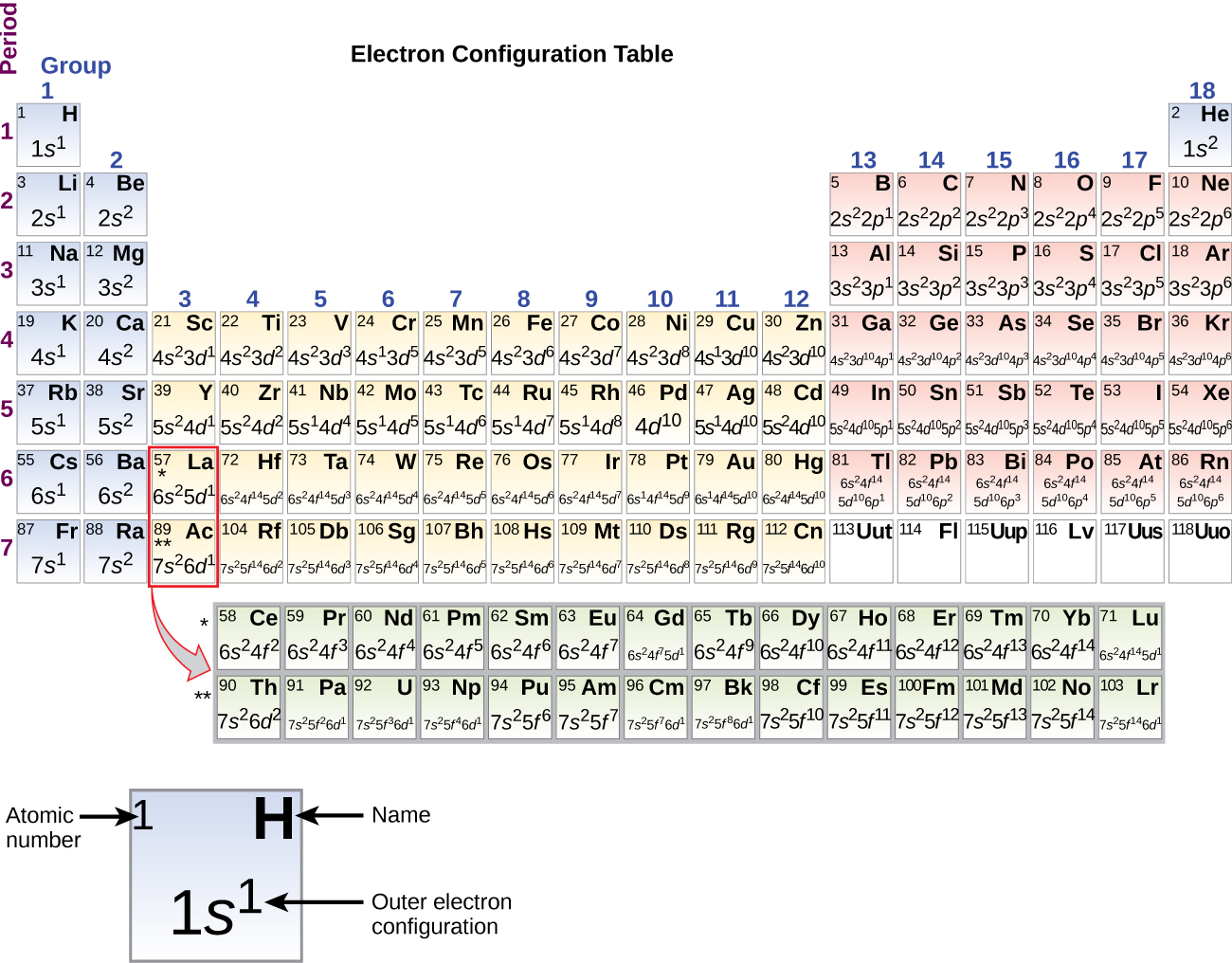
When we come to the next element in the periodic table we move down to period 4, group 1, the alkali metal potassium (atomic number 19). We might expect that we would begin to add electrons to the 3d subshell. However, all available chemical and physical evidence indicates that potassium is like lithium and sodium, and that the next electron is not added to the 3d level but is, instead, added to the 4s level since it is the next lowest energy level (Figure 10.5m). As discussed previously, the 3d orbital with no radial nodes is higher in energy because it is less penetrating and more shielded from the nucleus than the 4s, which has three radial nodes. Thus, potassium has an electron configuration of [Ar]4s1. Hence, potassium corresponds to its group 1 members, Li and Na in its valence shell configuration. The next element to consider is calcium. One electron is added to complete the 4s subshell and calcium has a
- complete electron configuration of 1s22s22p63s23p64s2 and
- noble gas electron configuration of [Ar]4s2
This gives calcium an outer-shell electron configuration corresponding to other elements in group 2 including beryllium and magnesium.
Beginning with the transition metal scandium (atomic number 21), additional electrons are added successively to the 3d subshell. This subshell is filled to its capacity with 10 electrons (remember that for l = 2 [d orbitals], there are 2l + 1 = 5 values of ml, meaning that there are five d orbitals that have a combined capacity of 10 electrons). The 4p subshell fills next. Note that for three series of elements, scandium (Sc) through copper (Cu), yttrium (Y) through silver (Ag), and lutetium (Lu) through gold (Au), a total of 10 d electrons are successively added to the (n – 1) shell next to the n shell to bring that (n – 1) shell from 8 to 18 electrons. For two series, lanthanum (La) through lutetium (Lu) and actinium (Ac) through lawrencium (Lr), 14 f electrons (l = 3, 2l + 1 = 7 ml values; thus, seven orbitals with a combined capacity of 14 electrons) are successively added to the (n – 2) shell to bring that shell from 18 electrons to a total of 32 electrons.
For a summary on electron configurations and orbital filling diagrams watch Electron Configuration (10min 16s).
Video Source: Bozemann Science. (2013, August 4). Electron configuration [Video]. YouTube.
Example 10.5a
Quantum Numbers and Electron Configurations
What is the electron configuration and orbital diagram for a phosphorus atom? What are the four quantum numbers for the last electron added?
Solution
The atomic number of phosphorus is 15. Thus, a phosphorus atom contains 15 electrons. The order of filling of the energy levels is 1s, 2s, 2p, 3s, 3p, 4s, . . . The 15 electrons of the phosphorus atom will fill up to the 3p orbital, which will contain three electrons:
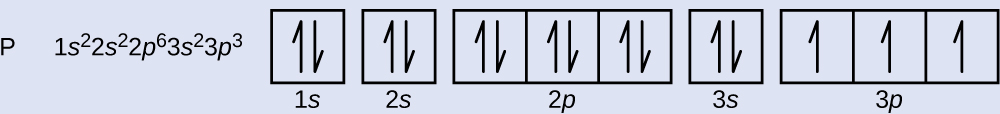
The last electron added is a 3p electron. Therefore, n = 3 and, for a p-type orbital, l = 1. The ml value could be –1, 0, or +1. The three p orbitals are degenerate, so any of these ml values is correct. For unpaired electrons, convention assigns the value of [latex]+\frac{1}{2}[/latex] for the spin quantum number; thus, [latex]m_s = +\frac{1}{2}[/latex].
Exercise 10.5a
Identify the atoms from the electron configurations given:
- [Ar]4s23d5
- [Kr]5s24d105p6
- 1s22s22p3
- 1s22s22p63s23p64s23d104p5
Check Your Answer[1]
Exceptions to Orbital Electron Filling Order
As mentioned previously in this section, the periodic table can be a powerful tool in predicting the electron configuration of an element. However, we do find exceptions to the order of filling of orbitals that are shown in Figure 10.5c or Figure 10.5d. For instance, the electron configurations (shown in Figure 10.5m) of the transition metals chromium (Cr; atomic number 24) and copper (Cu; atomic number 29), among others, are not those we would expect. In general, such exceptions involve subshells with very similar energy, and small effects can lead to changes in the order of filling.
In the case of Cr and Cu, we find that half-filled and completely filled subshells apparently represent conditions of preferred stability. This stability is such that an electron shifts from the 4s into the 3d orbital to gain the extra stability of a half-filled 3d subshell (in Cr) or a filled 3d subshell (in Cu). Other exceptions also occur. For example, niobium (Nb, atomic number 41) is predicted to have the electron configuration [Kr]5s24d3. Experimentally, we observe that its ground-state electron configuration is actually [Kr]5s14d4. We can rationalize this observation by saying that the electron–electron repulsions experienced by pairing the electrons in the 5s orbital are larger than the gap in energy between the 5s and 4d orbitals. There is no simple method to predict the exceptions for atoms where the magnitude of the repulsions between electrons is greater than the small differences in energy between subshells.
More about Electron Configurations and the Periodic Table
As described earlier, the periodic table arranges atoms based on increasing atomic number so that elements with the same chemical properties recur periodically. When their electron configurations are added to the table (Figure 10.5m), we also see a periodic recurrence of similar electron configurations in the outer shells of these elements. Because they are in the outer shells of an atom, valence electrons play the most important role in chemical reactions. The outer electrons have the highest energy of the electrons in an atom and are more easily lost or shared than the core electrons. Valence electrons are also the determining factor in some physical properties of the elements.
Elements in any one group (or column) have the same number of valence electrons; the alkali metals lithium and sodium each have only one valence electron, the alkaline earth metals beryllium and magnesium each have two, and the halogens fluorine and chlorine each have seven valence electrons. The similarity in chemical properties among elements of the same group occurs because they have the same number of valence electrons. It is the loss, gain, or sharing of valence electrons that defines how elements react.
It is important to remember that the periodic table was developed on the basis of the chemical behaviour of the elements, well before any idea of their atomic structure was available. Now we can understand why the periodic table has the arrangement it has—the arrangement puts elements whose atoms have the same number of valence electrons in the same group. This arrangement is emphasized in Figure 10.5m, which shows in periodic table form the electron configuration of the last subshell to be filled by the Aufbau principle. The coloured sections of Figure 10.5m show the three categories of elements classified by the orbitals being filled: main group, transition, and inner transition elements. These classifications determine which orbitals are counted in the valence shell, or highest energy level orbitals of an atom.
- Main group elements (sometimes called representative elements) are those in which the last electron added enters an s or a p orbital in the outermost shell, shown in blue and red in Figure 10.5m. This category includes all the nonmetallic elements, as well as many metals and the intermediate semi-metallic elements. The valence electrons for main group elements are those with the highest n level. For example, gallium (Ga, atomic number 31) has the electron configuration [Ar]4s23d104p1, which contains three valence electrons (underlined - 4s2, 4p1). The completely filled d orbitals count as core, not valence, electrons.
- Transition elements or transition metals. These are metallic elements in which the last electron added enters a d orbital. The valence electrons (those added after the last noble gas configuration) in these elements include the ns and (n – 1) d electrons. The official IUPAC definition of transition elements specifies those with partially filled d orbitals. Thus, the elements with completely filled orbitals (Zn, Cd, Hg, as well as Cu, Ag, and Au in Figure 10.5m) are not technically transition elements. However, the term is frequently used to refer to the entire d block (coloured yellow in Figure 10.5m), and we will adopt this usage in this textbook.
- Inner transition elements are metallic elements in which the last electron added occupies an f orbital. They are shown in green in Figure 10.5m. The valence shells of the inner transition elements consist of the (n – 2)f, the (n – 1)d, and the ns subshells. There are two inner transition series:
- The lanthanide series: lanthanide (La) through lutetium (Lu)
- The actinide series: actinide (Ac) through lawrencium (Lr)
Lanthanum and actinium, because of their similarities to the other members of the series, are included and used to name the series, even though they are transition metals with no f electrons.
Electron Configurations of Ions
We have seen that ions are formed when atoms gain or lose electrons. A cation (positively charged ion) forms when one or more electrons are removed from a parent (neutral) atom. For main group elements, the valence electrons that were added last are the first electrons removed. For transition metals and inner transition metals, however, valence electrons in the s orbital are easier to remove than the d or f electrons, and so the highest ns electrons are lost, and then the (n – 1)d or (n – 2)f electrons are removed. An anion (negatively charged ion) forms when one or more electrons are added to the valence shell of a parent atom. The added electrons fill in the order predicted by the Aufbau principle. Generally speaking:
- Metals forming simple cations typically lose valence electrons to achieve a stable electron configuration of their closest noble gas.
- Non-metals forming simple anions typically gain electrons to fill their outer valence shell to achieve a stable electron configuration of their closest noble gas.
Exercise 10.5b
Watch and Participate in this interactive video lesson (5min 11sec) to learn more about writing electron configurations of ions.
Check Your Learning Exercise (Text Version)
Question 1 (49 sec): For the two statements provided, fill in the [BLANK] with the correct key terms.
Key Terms:
- gain; 2. lose; 3. cation; 4. Anion
Statements:
- A positive ion is called a(n) [BLANK]. Atoms [BLANK] electrons to form this type of ion.
- A negative ion is called a(n) [BLANK]. Atoms [BLANK] electrons to form this type of ion.
Question 2 (2min 8sec): Which of the following statements about calcium are true?
- The electron configuration for neutral calcium atom is 1s22s22p63s23p64s2
- Calcium forms a Ca2+ cation by losing 2 electrons.
- The electron configuration for a calcium 2+ ion is 1s22s22p63s23p6
- a calcium 2+ ion has the same electron configuration as its closest noble gas, argon.
- All these options are correct statements.
Questions 3 (2min 54sec) is a statement that reads, “This Lewis dot diagram is introducing concepts in ionic bonding of simple ions and is discussed in more detail in chemical bonding units”
Question 4 (3min 42sec) is a statement that reads, “The electron configuration of Al is incorrectly written in the video. The correct electron configuration of Al is 1s22s22p63s23p1."
Question 5 (4min 7sec) is a statement that reads, “The three valence electrons lost from the aluminum atom were from 3s23p1."
Check Your Answer[2]
Activity Source: "Exercise 10.5b" by Jackie MacDonald is licensed under CC-BY-NC-SA 4.0, based on video source: Breslyn, W. (2020, October 1). How to write the electron configuration for ions [Video]. YouTube.
Example 10.5b
Predicting Electron Configurations of Ions
Write the electron configuration and orbital diagram of the following ions:
- O2-
- Na+
- P3–
- Al2+
- Fe2+
Solution
First, write out the electron configuration for each parent atom. We have shown full, unabbreviated configurations to provide more practice for students who want it, but listing the core-abbreviated electron configurations is also acceptable.
Next, determine whether an electron is gained or lost. Remember electrons are negatively charged, so ions with a positive charge have lost an electron. For main group elements, the last orbital gains or loses the electron. For transition metals, the last s orbital loses an electron before the d orbitals.
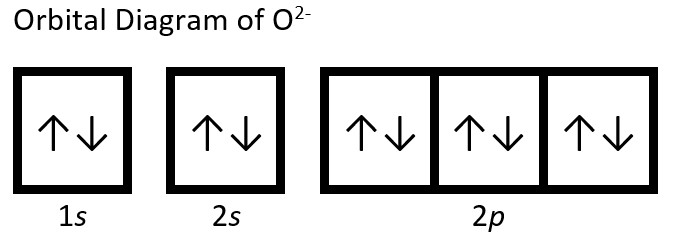
(a) O: 1s22s22p4. Oxygen anion gains two electrons in valence shell (2p shell), so O2-: 1s22s22p6.
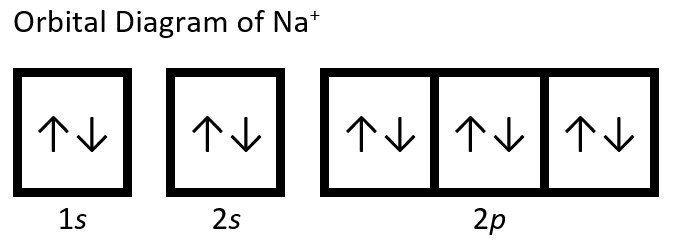
(b) Na: 1s22s22p63s1. Sodium cation loses one electron from valence shell (3s shell), so Na+: 1s22s22p6. To review a video showing the solution to this question watch Na+ Electron Configuration (Sodium Ion) (2min 17s)
Video Source: Breslyn, W. (2019, June 21). Na+ electron configuration (Sodium Ion) [Video]. YouTube.
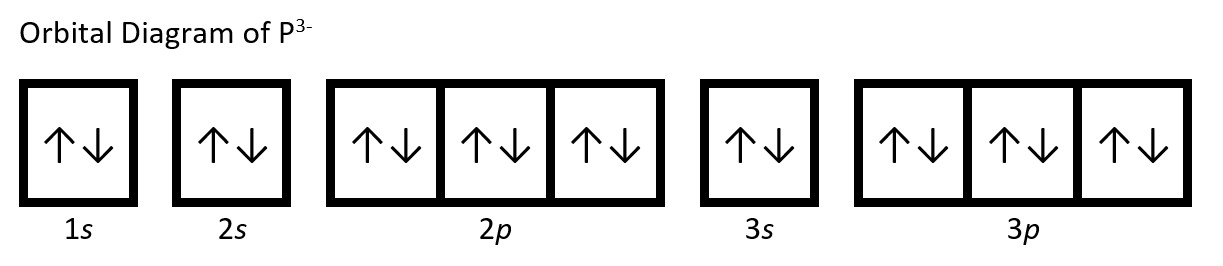
(c) P: 1s22s22p63s23p3. Phosphorus trianion gains three electrons (3 electrons are added to the valence shell, 3p) to form P3−: 1s22s22p63s23p6.
(d) Al: 1s22s22p63s23p1. Aluminum dication loses two electrons (from outer valence shells; one from 3p and the other from 3s) to form Al2+: 1s22s22p63s1.
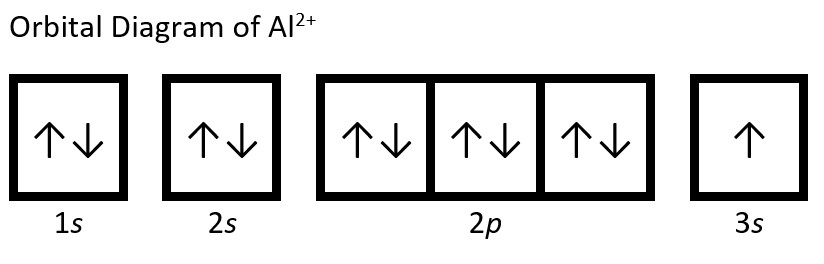
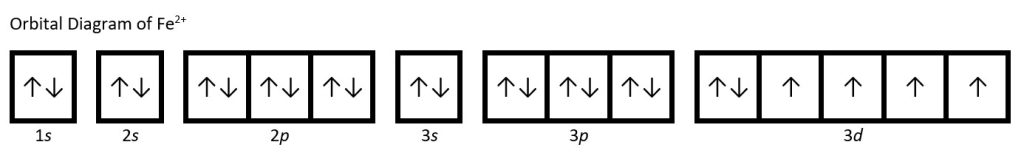
(e) Fe: 1s22s22p63s23p64s23d6. Iron(II) loses two electrons and, since it is a transition metal, they are removed from the 4s orbital: Fe2+: 1s22s22p63s23p63d6.
Exercise 10.5c
Predicting Electron Configurations of Ions
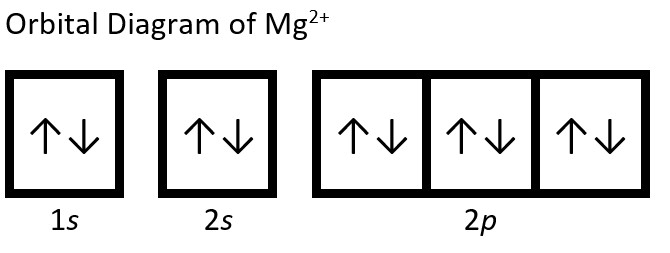
- Write the electron configuration and orbital diagram of the following ions:
- Mg2+
- Cl-
- Zn2+
- Which ion with a +2 charge has the electron configuration 1s22s22p63s23p63d104s24p64d5? Which ion with a +3 charge has this configuration?
Check Your Answer[3]
Links to Interactive Learning Tools
Explore The Dynamic Periodic Table of Elements from Ptabla to reference noble gas electron configurations of elements.
Practice Electron configuration order from eCampusOntario H5P Studio.
Explore the It's Elementary section of The Interactive Periodic Table from Annenberg Learner.
Practice Electron Configurations from the Physics Classroom.
Attribution & References
Except where otherwise noted, this page is adapted by Jackie MacDonald from:
- "3.4 Electronic Structure of Atoms (Electron Configurations)" In General Chemistry 1 & 2 by Rice University, a derivative of Chemistry (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson and is licensed under CC BY 4.0. Access for free at Chemistry (OpenStax) AND
- “6.4 Electronic Structure of Atoms (Electron Configurations)” In Chemistry 2e (Open Stax) by Paul Flowers, Klaus Theopold, Richard Langley & William R. Robinson is licensed under CC BY 4.0. Access for free at Chemistry 2e (Open Stax) . / Adaptations to content and addition of examples and exercises to optimize student comprehension.
- Orbital Diagrams of: O2- ion, Sodium Ion (Na+), Phosphorus 3- ion, Aluminum two plus ion (Al2+), Iron two plus ion (Fe2+) by Jackie MacDonald, licensed under the CC BY-NC-SA (Attribution NonCommercial ShareAlike) license
pictorial representation of the electron configuration showing each orbital as a box and each electron as an arrow
electronic structure of an atom in its ground state given as a listing of the orbitals occupied by the electrons
procedure in which the electron configuration of the elements is determined by “building” them in order of atomic numbers, adding one proton to the nucleus and one electron to the proper subshell at a time
Every orbital in a sublevel is singly occupied before any orbital is doubly occupied.
All of the electrons in singly occupied orbitals have the same spin (to maximize total spin).
electrons in the outermost or valence shell (highest value of n) of a ground-state atom; determine how an element reacts
electron in an atom that occupies the orbitals of the inner shells

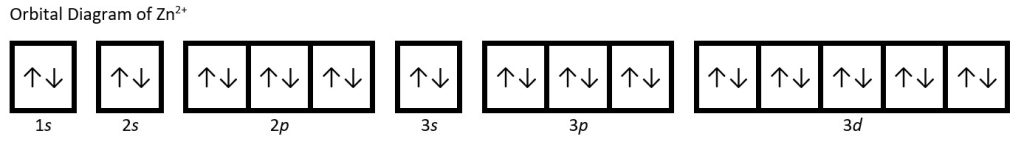 (1c) Zn2+: 1s22s22p63s23p63d10
(1c) Zn2+: 1s22s22p63s23p63d10
