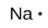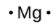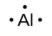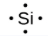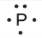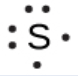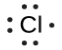9.1 – Atomic Properties of Chemical Bonds
Why are some substances chemically bonded molecules and others are an association of ions? The answer to this question depends upon the electronic structures of the atoms and nature of the chemical forces within the compounds – the topic of discussion in this chapter.
Types of Chemical Bonds
Although there are no sharply defined boundaries, chemical bonds are typically classified into three main types: ionic bonds, covalent bonds, and metallic bonds. In this chapter, each type of bond will be discussed and the general properties found in typical substances in which each bond type occurs.
Ionic bonds result from electrostatic forces that exist between ions of opposite charge. These bonds typically involve a metal atom bonding with a nonmetal atom.
Covalent bonds result from the sharing of electrons between two atoms. These bonds typically involve one nonmetallic element bonding with another.
Metallic bonds are found in solid metals (copper, iron, aluminum), where the metal atoms form a lattice structure and bonding electrons are free to move throughout the 3-dimensional structure.
In this text, ionic bonds are covered in Section 9.2 whereas covalent bonding is explained in Section 9.3. However, metallic bonding will be discussed in further detail in your future chemistry courses (such as CHM2353: Descriptive Inorganic Chemistry) and so will not be covered in this book.
Lewis Symbols
At the beginning of the 20th century, the American chemist G. N. Lewis (1875–1946) devised a system of symbols—now called Lewis electron dot symbols (often shortened to Lewis symbols) that can be used for predicting the number of bonds formed by most elements in their compounds. Each Lewis symbol consists of the chemical symbol for an element surrounded by dots that represent its valence electrons.
Lewis symbols:
are a convenient representation of valence electrons
allow you to keep track of valence electrons during bond formation
consist of the chemical symbol for the element plus a dot for each valence electron
To write an element’s Lewis symbol, we place dots representing its valence electrons, one at a time, around the element’s chemical symbol. Up to four dots are placed above, below, to the left, and to the right of the symbol (in any order, as long as elements with four or fewer valence electrons have no more than one dot in each position). The next dots, for elements with more than four valence electrons, are again distributed one at a time, each paired with one of the first four. For example, the electron configuration for atomic sulfur is [Ne]3s23p4, thus there are six valence electrons. Its Lewis symbol would therefore be:
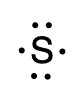
Fluorine, for example, with the electron configuration [He]2s22p5, has seven valence electrons, so its Lewis symbol is constructed as follows:
The number of dots in the Lewis symbol is the same as the number of valence electrons, which is the same as the last digit of the element’s group number in the periodic table. Lewis symbols for the elements in period 3 are given in Figure 9.1.1.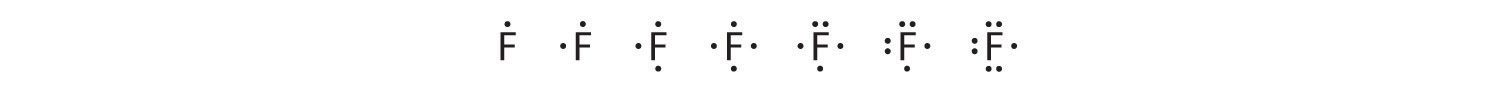
Lewis used the unpaired dots to predict the number of bonds that an element will form in a compound. Consider the symbol for phosphorus in Figure 9.1.1. The Lewis symbol explains why phosphorus, with three unpaired valence electrons, tends to form compounds in which it shares the unpaired electrons to form three bonds. Aluminum, which also has three unpaired valence electrons in its Lewis symbol, also tends to form compounds with three bonds, whereas silicon, with four unpaired valence electrons in its Lewis symbol, tends to share all of its unpaired valence electrons by forming compounds in which it has four bonds.
|
Atoms |
Electron Configuration |
Lewis Symbol |
|
sodium |
[Ne] 3s1 |
|
|
magnesium |
[Ne] 3s2 |
|
|
aluminum |
[Ne] 3s2 3p1 |
|
|
silicon |
[Ne] 3s2 3p2 |
|
|
phosphorus |
[Ne] 3s2 3p3 |
|
|
sulfur |
[Ne] 3s2 3p4 |
|
|
chlorine |
[Ne] 3s2 3p5 |
|
|
argon |
[Ne] 3s2 3p6 |
|
Figure 9.1.1. Lewis symbols illustrating the number of valence electrons for each element in the third period of the periodic table.
Symbol for an element or monatomic ion that uses a dot to represent each valence electron in the element or ion
Symbol for an element or monatomic ion that uses a dot to represent each valence electron in the element or ion