5.1 – Acid-Base Definitions & Conjugate Acid-Base Pairs
Acids and bases have been known for a long time. When Robert Boyle characterized them in 1680, he noted that acids dissolve many substances, change the colour of certain natural dyes (for example, they change litmus from blue to red), and lose these characteristic properties after coming into contact with alkalis (bases). In the eighteenth century, it was recognized that acids have a sour taste, react with limestone to liberate a gaseous substance (now known to be CO2), and interact with alkalis to form neutral substances. In 1815, Humphry Davy contributed greatly to the development of the modern acid-base concept by demonstrating that hydrogen is the essential constituent of acids. Around that same time, Joseph Louis Gay-Lussac concluded that acids are substances that can neutralize bases and that these two classes of substances can be defined only in terms of each other. The significance of hydrogen was reemphasized in 1884 when Svante Arrhenius defined an acid as a compound that dissolves in water to yield hydrogen cations (now recognized to be hydronium ions) and a base as a compound that dissolves in water to yield hydroxide anions.
Acids and bases are common solutions that exist everywhere. They have opposing chemical properties and are able to neutralize one another to form H2O, which will be discussed later in a subsection. Acids and bases can be defined by their physical and chemical observations in the table below.
Table 5.1.1. General Properties of Acids and Bases.
| Acids | Bases |
|
Produce a piercing pain in a wound |
Give a slippery feel |
|
Taste sour |
Taste bitter |
|
Are colourless when placed in phenolphthalein (an indicator) |
Are pink when placed in phenolphthalein (an indicator) |
|
Are red on blue litmus paper (a pH indicator) |
Are blue on red litmus paper (a pH indicator) |
|
Have a pH < 7 |
Have a pH > 7 |
|
Produce hydrogen gas when reacted with metals |
|
|
Produce carbon dioxide when reacted with carbonates |
|
|
Common examples: lemons, oranges, vinegar, urine, sulfuric acid, hydrochloric acid |
Common examples: soap, toothpaste, bleach, cleaning agents, limewater, ammonia water, sodium hydroxide |
Acids and bases in aqueous solutions will conduct electricity because they contain dissolved ions. Therefore, acids and bases are electrolytes, with strong acids and bases behaving as strong electrolytes versus weak acids and bases behaving as weak electrolytes.
In chemistry, acids and bases have been defined differently by three sets of theories: First is the Arrhenius definition, which relies on the idea that acids are substances that dissociate (break apart) in an aqueous solution to produce hydrogen (H+) ions while bases produce hydroxide (OH−) ions in solution. The other two definitions, discussed in detail in this chapter, include the Brønsted-Lowry definition, which defines acids as proton (H+) donors and bases as proton acceptors, and the Lewis Theory of acids and bases, which states that acids are electron pair acceptors while bases are electron pair donors. As shown in Figure 5.1.1, as the level of theory expands to include more substances covered under each definition, the closer the theory comes to accurately describing actual acid-base chemistry.
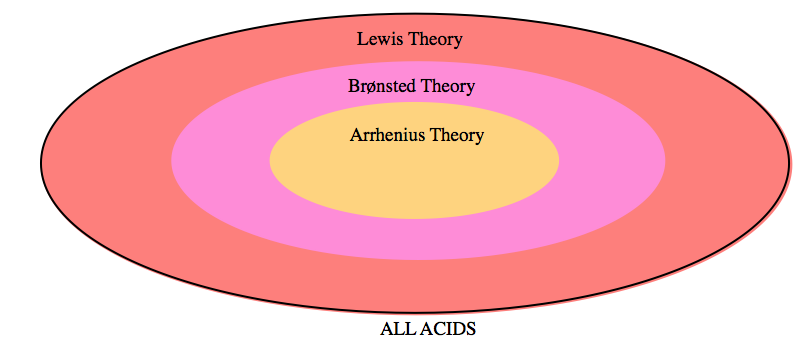
Figure 5.1.1. A Venn diagram representing the three levels of acid classification theory. Arrhenius Theory is the least accurate, and covers only a small subset of substances. Brønsted-Lowry expands upon Arrhenius’ definitions and thus is slightly broader. Lewis Theory, the most extensive explanation, most accurately describes all acid-base behaviour.
Arrhenius Definition of Acids and Bases
In 1884, the Swedish chemist Svante Arrhenius proposed two specific classifications of compounds, termed acids and bases. When dissolved in an aqueous solution, certain ions were released into the solution. The Arrhenius definition of acid-base reactions is a development of the “hydrogen theory of acids”. It was used to provide a modern definition of acids and bases, and followed from Arrhenius’s work with Friedrich Wilhelm Ostwald in establishing the presence of ions in aqueous solution in 1884. This led to Arrhenius receiving the Nobel Prize in Chemistry in 1903.
According to this theory, an Arrhenius acid is a compound that increases the concentration of H+ ions when added to water. This process is represented in what is known as a dissociation reaction in a chemical equation. For example:
HCl (aq) → H+ (aq) + Cl– (aq)
In this reaction, hydrochloric acid (HCl) dissociates (breaks apart) into hydrogen (H+) and chloride (Cl−) ions when dissolved in water, thereby releasing H+ ions into solution.
An Arrhenius base is a compound that increases the concentration of hydroxide ions, OH–, when added to water. For example:
NaOH (aq) → Na+ (aq) + OH– (aq)
In this reaction, an aqueous solution of sodium hydroxide (NaOH) dissociates into sodium ions (Na+) and hydroxide ions (OH–) when dissolved in water.
As you can imagine, this Arrhenius level of theory is quite limited. The Arrhenius definitions of acidity and alkalinity are restricted to aqueous solutions and refer to the concentration of the solvated ions. Under this definition, pure liquid H2SO4 or a solution of HCl dissolved in toluene would not be considered to be acidic, despite the fact that both of these acids are known proton donors. In addition, under the Arrhenius definition, a solution of sodium amide (NaNH2) in liquid ammonia is not alkaline, despite the fact that the amide ion (NH2−) will readily deprotonate ammonia. Thus, the Arrhenius definition can only describe acids and bases in an aqueous environment, and the Arrhenius theory excludes many substances known to display acid-base character.
Another flaw in Arrhenius’s theory is its reliance on the concept of dissociation of acids, i.e. an acid “HX” breaking apart in water to form H+ and X– ions. We now know that the H+ cation does not actually exist in this form in aqueous solution; it is more accurately depicted as the hydronium ion, H3O+.
Therefore, since the Arrhenius level of theory can only describe acids and bases in an aqueous environment, relies on the flawed concept of acid dissociation, and excludes many substances based on its restrictive definitions, this theory can generally be disregarded moving forward in our discussion.
Brønsted-Lowry Definition of Acids and Bases
In 1923, the Danish chemist Johannes Brønsted and the English chemist Thomas Lowry expanded upon Arrhenius’s ideas by broadening the definitions of acids and bases. The Brønsted-Lowry theory centers on the proton, H+. A proton is what remains when the most common isotope of hydrogen, 1H, loses an electron. A compound that donates a proton to another compound is called a Brønsted-Lowry acid, and a compound that accepts a proton is called a Brønsted-Lowry base. An acid-base reaction is thus defined as the transfer of a proton from a proton donor (acid) to a proton acceptor (base).
Using these very simple definitions, the Brønsted-Lowry level of theory covers the large majority of known acid-base behaviour, and thus this theory can be successfully applied for most of this chapter (and this course). However, to be inclusive to all acids and bases, an even more general theory is required. In Section 5.6 of this text, we will introduce the final and most general model of acid-base behavior introduced by the American chemist G. N. Lewis.
Acids may be inorganic or organic substances (e.g. HCl vs acetic acid, CH3COOH), neutral substances or ions (e.g. H2O vs HSO4−). Each of these species contains at least one hydrogen atom; to be defined as a Brønsted-Lowry acid, the substance must be able to donate this atom to another substance in the form of a proton, H+. Bases may also be inorganic or organic substances (e.g. NaOH vs methyl amine, CH3NH2), neutral substances or ions (e.g. NH3 vs [Al(H2O)5OH]2+). Each of these examples are capable of accepting a proton and may therefore be classified as Brønsted-Lowry bases. The most familiar and recognizable bases tend to be inorganic ionic compounds which contain the hydroxide ion, such as NaOH and Ca(OH)2.
The conjugate base of an acid is what remains after the acid has donated a proton. This species is a base because it can accept a proton to re-form the original acid:
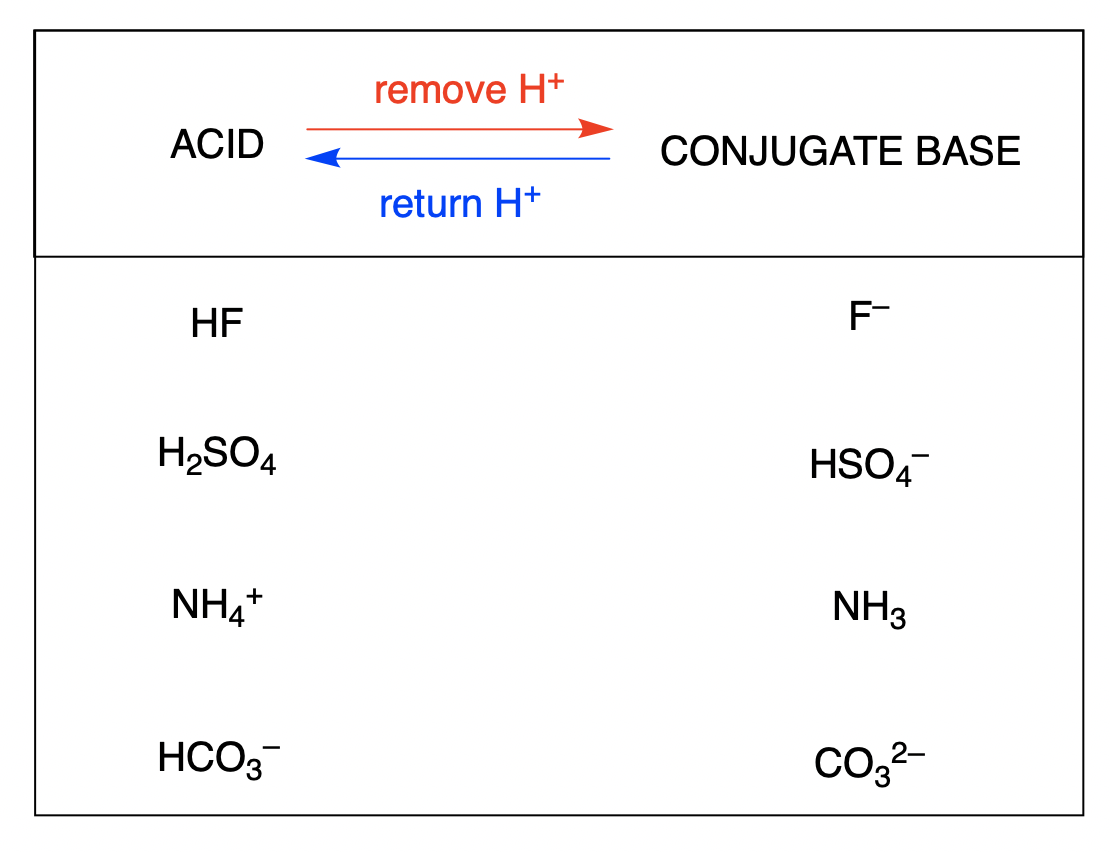
The conjugate acid of a base is what results after the base has accepted a proton. This species is an acid because it can give up a proton to re-form the original base:

In the two schemes above, the behaviour of acids as proton donors and bases as proton acceptors are represented in isolation. In reality, all acid-base reactions involve the transfer of protons between acids and bases. This is quite useful when tasked with exercises asking you to classify reactions, such as those done in Section 1.4 – Solution Stoichiometry when we discussed the three types of chemical reactions covered in CHM 1311: acid-base, redox and precipitation reactions. If you can identify all four terms in a proton-transfer reaction (an acid, a base, a conjugate acid, and a conjugate base), you can be certain that it is a Brønsted-Lowry acid-base reaction. For example, consider the acid-base reaction that takes place when ammonia is dissolved in water. A water molecule (functioning as an acid) transfers a proton to an ammonia molecule (functioning as a base), yielding the conjugate base of water, OH−, and the conjugate acid of ammonia, NH4+:
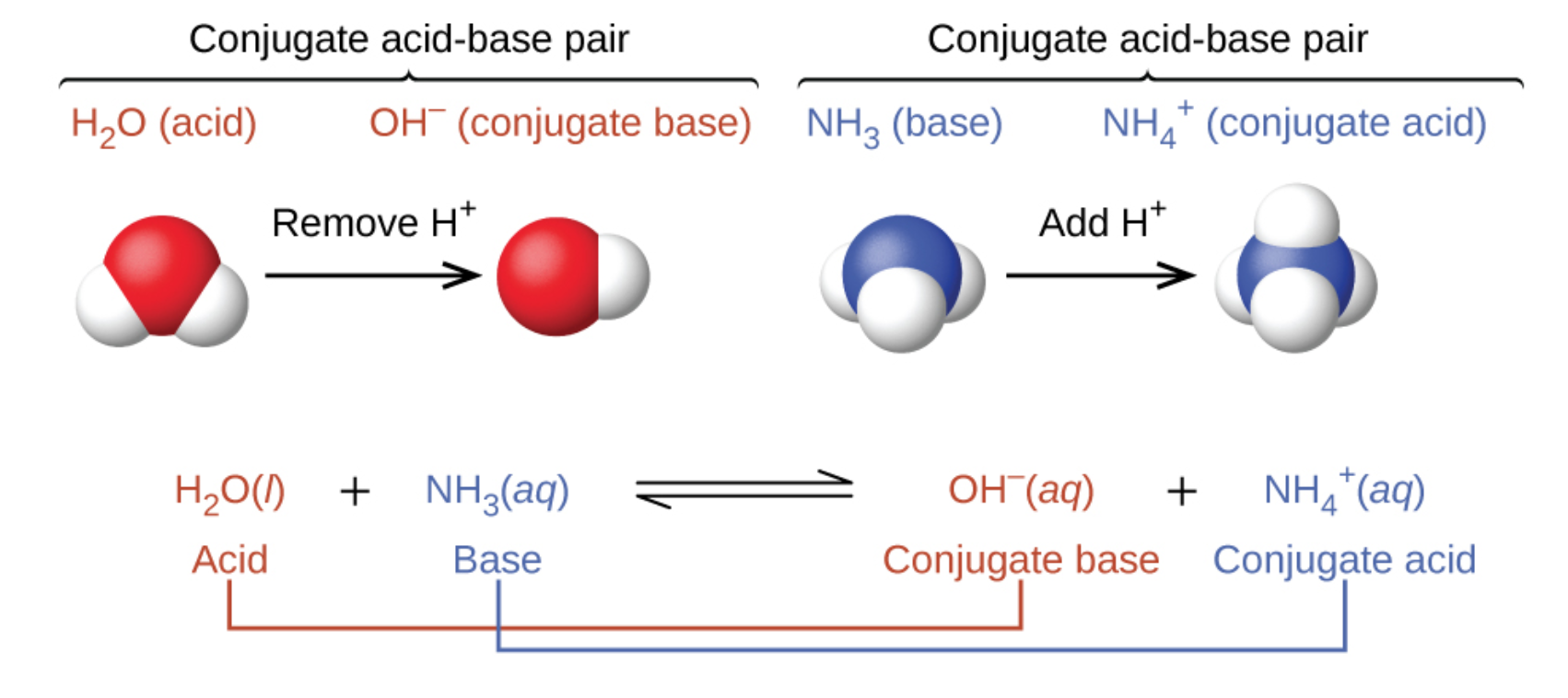
The reaction between a Brønsted-Lowry acid and base is called ionization (note how this differs slightly from the term dissociation defined previously). More specifically, when we add an acid to water, an acid ionization occurs, in which protons are transferred from the acid molecules to the water molecules. For example, when hydrogen fluoride dissolves in water and ionizes, protons are transferred from hydrogen fluoride molecules to water molecules, yielding hydronium ions and fluoride ions:
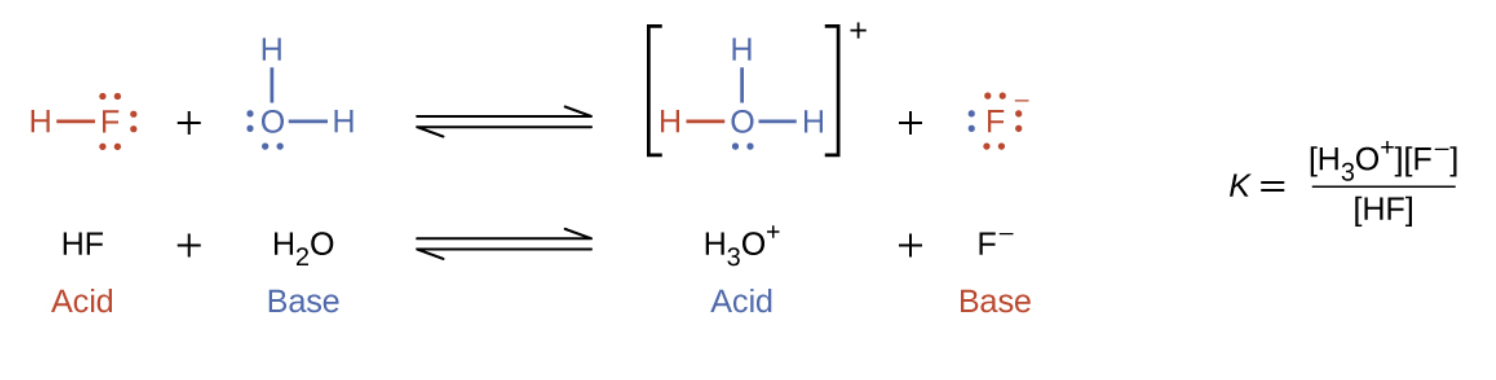
When we add a base to water, a base ionization reaction occurs in which protons are transferred from water molecules to base molecules. For example, adding pyridine to water yields hydroxide ions and pyridinium ions:
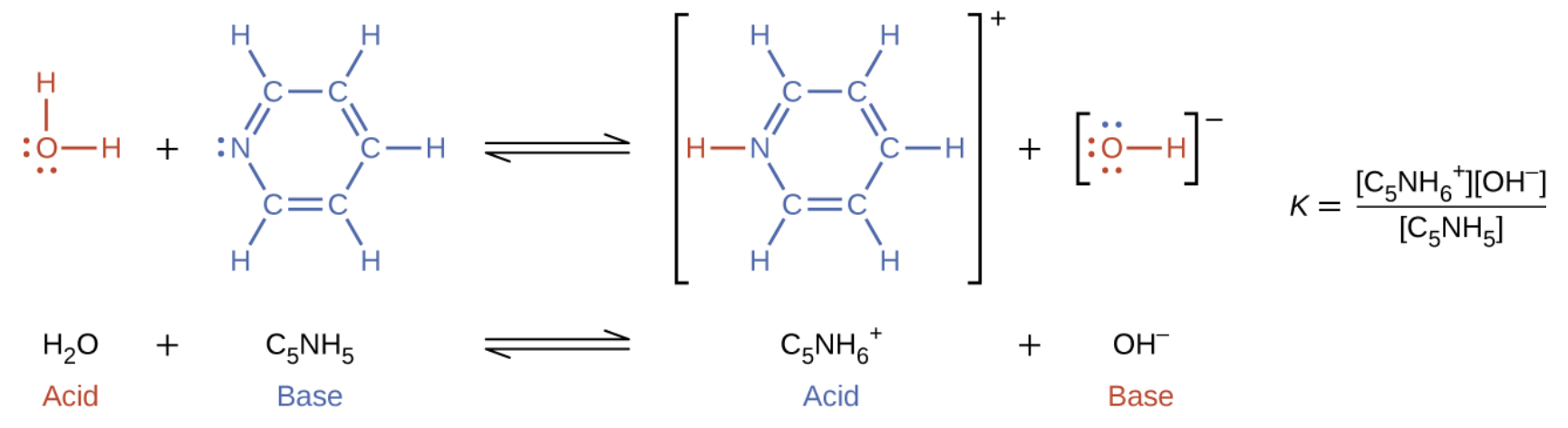
Notice that both these ionization reactions are represented as equilibrium processes. The relative extent to which these acid and base ionization reactions proceed is an important topic treated in a later section of this chapter.
|
Since an acid-base reaction starts with two species and finishes two species, we should not use the term “dissociation” in the context of acids and bases (e.g. the dissociation of an acid in solution). The reason is because the term implies that one species dissociates into two, while in reality the acid-base reaction involves a proton transfer between two species. |
Often when describing an acid-base reaction, we can refer to the portion of acid or base that does not ionize in solution (i.e. it remains in its neutral form) as either nonionized or unionized. In this text, we will use the latter term, but be prepared to recognize both adjectives when studying chemistry.
How can you spot the difference between a chemist and a plumber? Ask them to pronounce the word UNIONIZED!
Questions
★ Questions
1. Write equations that show NH3 as both a conjugate acid and a conjugate base.
2. Write equations that show H2PO4− acting both as an acid and as a base.
3. Show by suitable net ionic equations that each of the following species can act as a Brønsted-Lowry acid:
(a) H3O+
(b) HCl
(c) NH3
(d) CH3CO2H
(e) NH4+
(f) HSO4–
4. Show by suitable net ionic equations that each of the following species can act as a Brønsted-Lowry acid:
(a) HNO3
(b) PH4+
(c) H2S
(d) CH3CH2COOH
(e) H2PO4–
(f) HS–
5. Show by suitable net ionic equations that each of the following species can act as a Brønsted-Lowry base:
(a) HS–
(b) PO43-
(c) NH2–
(d) C2H5OH
(e) O2-
(f) H2PO4–
6. What is the conjugate acid of each of the following? What is the conjugate base of each?
(a) H2S
(b) H2PO4–
(c) PH3
(d) HS–
(e) HSO3–
(f) H3O2+
(g) H4N2
(h) CH3OH
7. Identify and label the Brønsted-Lowry acid, its conjugate base, the Brønsted-Lowry base, and its conjugate acid in each of the following equations:
(a) HNO3 + H2O → H3O+ + NO3–
(b) CN– + H2O → HCN + OH–
(c) H2SO4 + Cl– → HCl + HSO4–
(d) HSO4– + OH– → SO42- + H2O
(e) O2– + H2O → 2 OH–
(f) [Cu(H2O)3(OH)]+ + [Al(H2O)6]3+ → [Cu(H2O)4]2+ + [Al(H2O)5(OH)]2+
(g) H2S + NH2– → HS– + NH3
Answers
1. One example for NH3 as a conjugate acid: NH2– + H+ → NH3; as a conjugate base: NH4+ (aq) + OH– (aq) → NH3 (aq) + H2O (l)
2. As an acid: H2PO4– + H+ → H3PO4 ; as a base: H2PO4– + OH– → HPO42- + H2
3. (a) H3O+ (aq) → H+ (aq) + H2O (l)
(b) HCl (l) → H+ (aq) + Cl– (aq)
(c) NH3 → NH2– + H+
(d) CH3CO2H (aq) → H+ (aq) + CH3CO2– (aq)
(e) NH4+ (aq) → NH3 (aq) + H+ (aq)
(f) HSO4– (aq) → H+ (aq) + SO42- (aq)
4. (a) HNO3 → H+ + NO3–
(b) PH4+ → H+ + PH3
(c) H2S → H+ + HS–
(d) CH3CH2COOH → H+ + CH3CH2COO–
(e) H2PO4– → H+ + HPO42-
(f) HS– → H+ + S2-
5. (a) H2O (l) + H+ (aq) → H3O+ (aq)
(b) OH– (aq) + H+ (aq) → H2O (l)
(c) NH3 (aq) + H+ (aq) → NH4+ (aq)
(d) CN– (aq) + H+ (aq) → HCN (aq)
(e) S2- (aq) + H+ (aq) → HS– (aq)
(f) H2PO4– (aq) + H+ (aq) → H3PO4 (aq)
6. (a) Conjugate acid: H3S+ ; Conjugate base: HS–
(b) Conjugate acid: H3PO4 ; Conjugate base: HPO42-
(c) Conjugate acid: PH4+ ; Conjugate base: PH2–
(d) Conjugate acid: H2S ; Conjugate base: S2-
(e) Conjugate acid: H2SO3 ; Conjugate base: SO32-
(f) Conjugate acid: H4O22+ ; Conjugate base: H2O2
(g) Conjugate acid: H5N2+ ; Conjugate base: H3N2–
(h) Conjugate acid: CH3OH2+ ; Conjugate base: CH3O–
7. The labels are Bronsted-Lowry acid = BA; its conjugate base = CB; Bronsted-Lowry Base = BB; its conjugate acid = CA.
(a) HNO3 (BA), H2O (BB), H3O+ (CA), NO3– (CB)
(b) CN– (BB), H2O (BA), HCN (CA), OH– (CB)
(c) H2SO4 (BA), Cl– (BB), HCl (CA), HSO4– (CB)
(d) HSO4– (BA), OH– (BB), SO42- (CB), H2O (CA)
(e) O2- (BB), H2O (BA), OH– (CA and CB)
(f) [Cu(H2O)3(OH)]+ (BB), [Al(H2O)6]3+ (BA), [Cu(H2O)4]2+ (CA), [Al(H2O)5(OH)]2+ (CB)
(g) NH2– (BB), HS– (CB), NH3 (CA)
Compound that increases the hydrogen ion concentration in aqueous solution
Compound that increases the hydroxide ion concentration in aqueous solution
Any species that can donate a proton to another molecule
Any species that can accept a proton from another molecule
Substance formed when an acid loses a proton
Substance formed when a base gains a proton
Reaction involving the transfer of a proton from an acid to water, yielding hydronium ions and the conjugate base of the acid
Reaction involving the transfer of a proton from water to a base, yielding hydroxide ions and the conjugate acid of the base

