8.6 – General Atomic Properties
In many cases, elements that belong to the same group (vertical column) on the periodic table form ions with the same charge because they have the same number of valence electrons. Thus, the periodic table becomes a tool for remembering the charges on many ions. For example, all ions made from alkali metals, the first column on the periodic table, have a 1+ charge. Ions made from alkaline earth metals, the second group on the periodic table, have a 2+ charge. On the other side of the periodic table, the next-to-last column, the halogens, form ions having a 1− charge. Figure 8.6.1. shows how the charge on many ions can be predicted by the location of an element on the periodic table. Note the convention of first writing the number and then the sign on a multiply charged ion. The barium cation is written Ba2+, not Ba+2.
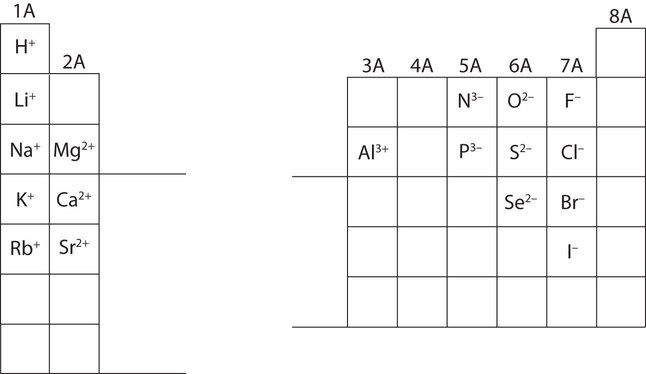
Figure 8.6.1. Predicting Ionic Charges. The charge that an atom acquires when it becomes an ion is related to the structure of the periodic table. Within a group (family) of elements, atoms form ions of a certain charge.
Magnetic Properties
The magnetic moment of a system measures the strength and the direction of its magnetism. The term itself usually refers to the magnetic dipole moment. Anything that is magnetic, like a bar magnet or a loop of electric current, has a magnetic moment. A magnetic moment is a vector quantity, with a magnitude and a direction. An electron has an electron magnetic dipole moment, generated by the electron’s intrinsic spin property, making it an electric charge in motion. There are many different magnetic forms: including paramagnetism, and diamagnetism, ferromagnetism, and anti-ferromagnetism. Only paramagnetism and diamagnetism are discussed here.
Paramagnetism
Paramagnetism refers to the magnetic state of an atom with one or more unpaired electrons. The unpaired electrons are attracted by a magnetic field due to the electrons’ magnetic dipole moments. Hund’s Rule states that electrons must occupy every orbital singly before any orbital is doubly occupied. This may leave the atom with many unpaired electrons. Because unpaired electrons can orient in either direction, they exhibit magnetic moments that can align with a magnet. This capability allows paramagnetic atoms to be attracted to magnetic fields. Diatomic oxygen, O2 is a good example of paramagnetism (that is best understood with molecular orbital theory). The following video shows liquid oxygen attracted into a magnetic field created by a strong magnet.
As shown in the video, since molecular oxygen, O2, is paramagnetic, it is attracted to the magnet. In contrast, molecular nitrogen, N2, has no unpaired electrons and it is diamagnetic (discussed below); it is therefore unaffected by the magnet.
Note: Paramagnetism is a form of magnetism whereby materials are attracted by an externally applied magnetic field.
There are some exceptions to the paramagnetism rule; these concern some transition metals, in which the unpaired electron is not in a d-orbital. Examples of these metals include Sc3+, Ti4+, Zn2+, and Cu+. These metals are not defined as paramagnetic: they are considered diamagnetic because all d-electrons are paired. Paramagnetic compounds sometimes display bulk magnetic properties due to the clustering of the metal atoms. This phenomenon is known as ferromagnetism, but this property is not discussed here.
Diamagnetism
Diamagnetic substances are characterized by paired electrons—except in the previously-discussed case of transition metals, there are no unpaired electrons. According to the Pauli Exclusion Principle which states that no two identical electrons may take up the same quantum state at the same time, the electron spins are oriented in opposite directions. This causes the magnetic fields of the electrons to cancel out; thus there is no net magnetic moment, and the atom cannot be attracted into a magnetic field. In fact, diamagnetic substances are weakly repelled by a magnetic field as demonstrated with the pyrolytic carbon sheet in Figure 8.6.2.

Figure 8.6.2. Levitating pyrolytic carbon: A small (~6 mm) piece of pyrolytic graphite levitating over a permanent neodymium magnet array (5 mm cubes on a piece of steel). Note that the poles of the magnets are aligned vertically and alternate (two with north facing up, and two with south facing up, diagonally). Image used with permission from Wikipedia.
Note: diamagnetic materials are repelled by the applied magnetic field.
Diamagnetism, to a greater or lesser degree, is a property of all materials and always makes a weak contribution to the material’s response to a magnetic field. For materials that show some other form of magnetism (such paramagnetism), the diamagnetic contribution becomes negligible.
How to Tell if a Substance is Diamagnetic or Paramagnetic
The magnetic form of a substance can be determined by examining its electron configuration: if it shows unpaired electrons, then the substance is paramagnetic; if all electrons are paired, the substance is diamagnetic. This process can be broken into four steps:
Find the electron configuration
Draw the valence orbitals
Look for unpaired electrons
Determine whether the substance is paramagnetic (one or more electrons unpaired) or diamagnetic (all electrons paired)
Example 8.6.1 – Chlorine Atoms
Are chlorine atoms paramagnetic or diamagnetic?
Solution
Step 1: Find the electron configuration
For Cl atoms, the electron configuration is 3s23p5
Step 2: Draw the valence orbitals
Ignore the core electrons and focus on the valence electrons only.
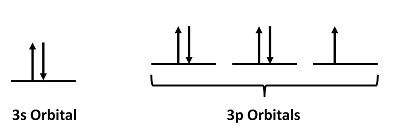
Step 3: Look for unpaired electrons
There is one unpaired electron.
Step 4: Determine whether the substance is paramagnetic or diamagnetic
Since there is an unpaired electron, Cl atoms are paramagnetic (but weakly since only one electron is unpaired).
Check Your Learning 8.6.1 – Chlorine Atoms
Indicate whether boron atoms are paramagnetic or diamagnetic.
Answer
The B atom has 2s22p1 as the electron configuration. Because it has one unpaired electron, it is paramagnetic.
Example 8.6.2 – Zinc Atoms
Are zinc atoms paramagnetic or diamagnetic?
Solution
Step 1: Find the electron configuration
For Zn atoms, the electron configuration is 4s23d10
Step 2: Draw the valence orbitals
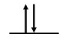
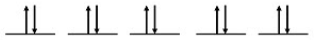
4s Orbital
3d Orbitals
Step 3: Look for unpaired electrons
There are no unpaired electrons.
Step 4: Determine whether the substance is paramagnetic or diamagnetic
Because there are no unpaired electrons, Zn atoms are diamagnetic.
Check Your Learning 8.6.2 – Zinc Atoms
Indicate whether F– ions are paramagnetic or diamagnetic.
Answer
The F– ion has 2s22p6 as the electron configuration. Because it has no unpaired electrons, it is diamagnetic.
★ Questions
1. Can a molecule with an odd number of electrons ever be diamagnetic? Explain why or why not.
2. Which of the period 2 homonuclear diatomic molecules are predicted to be paramagnetic?
3. How many unpaired electrons are found in oxygen atoms ?
4. How many unpaired electrons are found in bromine atoms?
5. Indicate whether Fe2+ ions are paramagnetic or diamagnetic.
Answers
1. An odd number of electrons can never be paired, regardless of the arrangement of the molecular orbitals. It will always be paramagnetic.
2. Oxygen
3. The O atom has 2s22p4 as the electron configuration. Therefore, O has 2 unpaired electrons.
4. The Br atom has 4s23d104p5 as the electron configuration. Therefore, Br has 1 unpaired electron.
5. The Fe2+ ion has 3d6 as the electron configuration. Because it has 4 unpaired electrons, it is paramagnetic.
Measure of the magnetic strength and direction of a system; a vector quantity
Measure of the magnetic strength and direction of a system; a vector quantity
Magnetic state of substances characterized by one or more unpaired electrons; paramagnetic substances are attracted by an externally applied magnetic field
Magnetic state of substances characterized by paired electrons; diamagnetic substances are (weakly) repelled by an applied magnetic field

