5.5 – Hydrolysis of Salt Solutions
As we have seen in the section on chemical reactions, when an acid and base are mixed, they undergo a neutralization reaction. The word “neutralization” seems to imply that a stoichiometrically equivalent solution of an acid and a base would have a neutral pH. This is sometimes true, but the salts that are formed in these reactions may have acidic or basic properties of their own, as we shall now see.
Acid-Base Neutralization
When we mix solutions of an acid and a base, an acid-base neutralization reaction occurs. All acid-base neutralization reactions are assumed to be 100% complete for stoichiometric purposes. For example, consider the following acid-base reaction:
HCl (aq) + NaOH (aq) → NaCl (aq) + H2O (l)
Note the one-way arrow, indicating that this is a heavily product-favoured reaction. Why do we not use an equilibrium arrow instead? Let’s breakdown the above reaction into its individual steps to explain:
HCl ionizes completely in water. This means there is no actual HCl in the solution, it completely dissolves into H3O+ and Cl–:
HCl (aq) → H3O+ (aq) + Cl–(aq)
NaOH also ionizes completely in water and leaves only Na+ and OH– in solution:
NaOH (aq) → Na+ (aq) + OH–(aq)
In the acid-base neutralization, the products of Step 1 react with the products of Step 2, to yield the following full ionic equation:
H3O+ (aq) + Cl–(aq) + Na+ (aq) + OH–(aq) → 2H2O (l) + Cl–(aq) + Na+(aq)
Once the spectator ions are removed, you get the net ionic equation:
H3O+ (aq) + OH–(aq) → 2H2O (l)
Notice that this is the reverse of the autoionization of water, meaning K = 1/Kw = 1014. This K value is so large that the reaction is assumed to go to completion, allowing for a one way arrow to be drawn.
Application of Acid-Base Neutralization 1: Stomach Antacids
Our stomachs contain a solution of roughly 0.03 M HCl, which helps us digest the food we eat. The burning sensation associated with heartburn is a result of the acid of the stomach leaking through the muscular valve at the top of the stomach into the lower reaches of the esophagus. The lining of the esophagus is not protected from the corrosive effects of stomach acid the way the lining of the stomach is, and the results can be very painful. When we have heartburn, it feels better if we reduce the excess acid in the esophagus by taking an antacid. As you may have guessed, antacids are bases. One of the most common antacids is calcium carbonate, CaCO3. The reaction,
CaCO3 (aq) + 2HCl (aq) → CaCl2 (aq) + H2O (l) +CO2 (g)
not only neutralizes stomach acid, it also produces CO2(g), which may result in a satisfying belch.
Application of Acid-Base Neutralization 2: Culinary Aspects of Chemistry
Cooking is essentially synthetic chemistry that happens to be safe to eat. There are a number of examples of acid-base chemistry in the culinary world. One example is the use of baking soda, or sodium bicarbonate, in baking. NaHCO3 is a base. When it reacts with an acid such as lemon juice, buttermilk, or sour cream in a batter, bubbles of carbon dioxide gas are formed from decomposition of the resulting carbonic acid, and the batter “rises.” Baking powder is a combination of sodium bicarbonate, and one or more acid salts that react when the two chemicals come in contact with water in the batter.
Many people like to put lemon juice or vinegar, both of which are acids, on cooked fish (Figure 5.5.1). It turns out that fish have volatile amines (bases) in their systems, which are neutralized by the acids to yield involatile ammonium salts. This reduces the odour of the fish, and also adds a “sour” taste that we seem to enjoy.
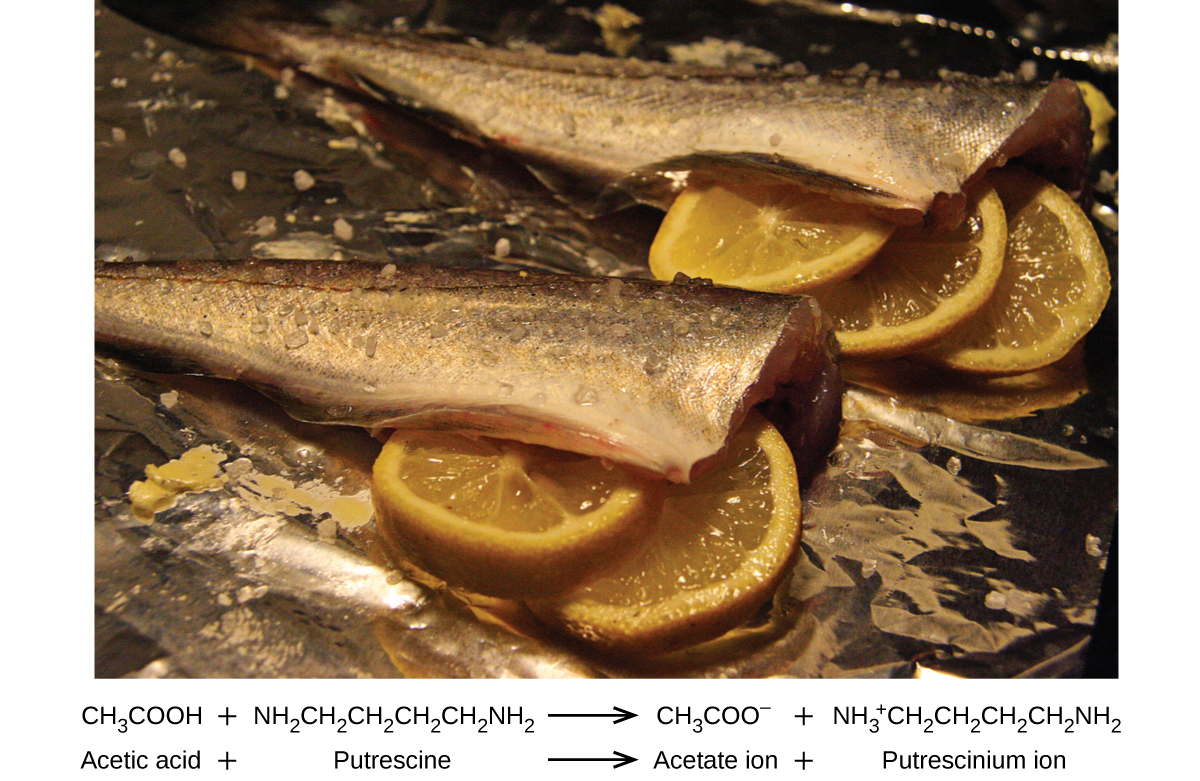
Figure 5.5.1. A neutralization reaction takes place between citric acid in lemons or acetic acid in vinegar, and the bases in the flesh of fish.
Pickling is a method used to preserve vegetables using a naturally produced acidic environment. The vegetable, such as a cucumber, is placed in a sealed jar submerged in a brine solution. The brine solution favours the growth of beneficial bacteria and suppresses the growth of harmful bacteria. The beneficial bacteria feed on starches in the cucumber and produce lactic acid as a waste product in a process called fermentation. The lactic acid eventually increases the acidity of the brine to a level that kills any harmful bacteria, which require a basic environment. Without the harmful bacteria consuming the cucumbers they are able to last much longer than if they were unprotected. A byproduct of the pickling process changes the flavour of the vegetables with the acid making them taste sour.
Acid-base neutralization reactions can be summarized by the following general chemical equation:
acid (aq) + base (aq) → salt (aq) + water
where the driving force of the reaction (why we use a one-way arrow) is the formation of water. Again, the term neutralization implies that the product, an aqueous solution of an ionic salt, has a neutral pH. However, even if we mix stoichiometrically equivalent quantities, we sometimes may find that the resulting solution is not neutral. It could contain either an excess of hydronium ions or an excess of hydroxide ions because the nature of the salt formed determines whether the solution is acidic, neutral, or basic.
Example 5.5.1 – Predicting the salts formed in acid-base neutralizations
Predict the salt produced when the following neutralizations go to completion:
(a) HCOOH and NaOH
(b) HCN and NH3
(c) HCl and CH3NH2
Solution
(a) HCOOH is a weak acid and NaOH is a strong base. The resulting salt will be HCOONa.
(b) HCN is a weak acid and NH3 is a weak base. The resulting salt will be NH4CN.
(c) HCl is a strong acid and CH3NH2 is a weak base. The resulting salt will be CH3NH2Cl.
Check Your Learning 5.5.1 – Predicting the salts formed in acid-base neutralizations
Predict the salt produced when the following neutralizations go to completion.
(a) RbOH and HCl
(b) HNO2 and NaOH
(c) CH3COOH and NH3
(d) NH3 and HCl
(e) HI and CH3NH2
Answer
(a) RbCl
(b) NaNO2
(c) NH4CH3COO
(d) NH4Cl
(e) CH3NH3I
Hydrolysis of Salts
As a general term in chemistry, the word hydrolysis refers to cleavage of a bond (-lysis) by the action of water (hydro-). In the context of ionic salts, they may hydrolyze in water to produce either acidic or basic aqueous solutions, depending on the nature of the salt. Consider a generic ionic compound, XY (s). This salt is dissolved in water and ionizes to form a cation, X+ (aq) and an anion, Y– (aq). By looking at the charges on the ions, we see that, if hydrolysis occurs, the cation produces H3O+ ion and the anion produces OH– ion (Figure 5.5.2).
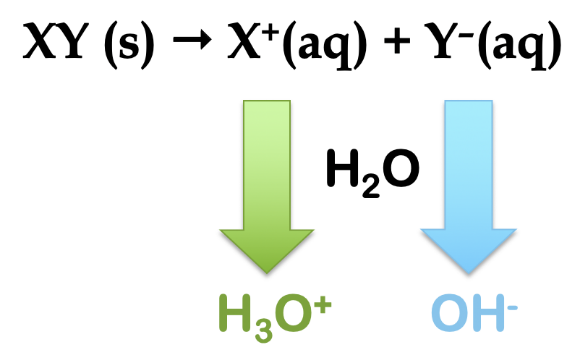
Figure 5.5.2. The general reaction scheme for the hydrolysis of a salt, XY. If reaction with water occurs, the cation produces hydronium ion and the anion produces hydroxide ion.
Therefore, we can envision 4 possible scenarios for the hydrolysis of any salt, XY:
- If X+ hydrolyzes but Y– doesn’t, then H3O+ is produced and the solution is ACIDIC.
- If Y– hydrolyzes but X+ doesn’t, then OH– is produced and the solution is BASIC.
- If neither X+ nor Y– hydrolyzes, then there is NO CHANGE to the solution’s pH.
- If both X+ and Y– hydrolyze, then the change to the solution’s pH cannot immediately be determined (we will need to perform further calculations).
Let us examine first how and when salts ionize, and then look at examples of salts from each of the above 4 scenarios.
Conjugate Acid-Base Pairs and Hydrolysis
As discussed in previous sections, in conjugate acid-base pairs, if one species is strong, the other is comparatively weak; for example, a strong acid (very good proton donor) has a weak conjugate base (very poor proton acceptor). As we saw, this leads to acid-base equilibria proceeding in preferred directions, from the stronger acid and base to the weaker acid and base. Let’s use HCl again as an example: it is a very strong acid and ionizes completely in water:
HCl (aq) + H2O (aq) → H3O+ (aq) + Cl– (aq)
In this reaction, HCl is a strong acid (an excellent proton donor), making its conjugate base, Cl–, a very poor proton acceptor. This means that, if we were to add the conjugate base, Cl– to water instead, we would NOT expect it to behave as a Brønsted-Lowry base: it would NOT react with water to pick up a proton to produce OH–:
Cl– (aq) + H2O (aq) ⇌ HCl (aq) + OH– (aq)
The above reaction is pure nonsense: we know that HCl is a powerful proton donor and thus would not remain in solution (it would immediately react with the generated OH– to return to the reactants). In other words, since Cl– is the weak conjugate base of a strong acid, it does not hydrolyze (react with water).
Let’s now compare this result to that of acetic acid, CH3COOH, a weak acid that only partially ionizes in solution:
CH3COOH (aq) + H2O (aq) ⇆ H3O+ (aq) + CH3COO– (aq)
As we have seen, this is reactant-favoured, meaning that at equilibrium, the majority of the acid prefers to be in its neutral, unionized form. Since CH3COOH is a weak acid (poor proton donor) we must conclude that its conjugate base, CH3COO–, must be a good base (good proton acceptor). Therefore, if we were to add acetate ion directly to water instead, we WOULD expect it to behave as a Brønsted-Lowry base: it would react with water to pick up a proton to produce OH–:
CH3COO– (aq) + H2O (aq)⇆ CH3COOH (aq) + OH– (aq)
Since we know that acetic acid prefers to be in its neutral, unionized form, we can expect that the above reaction occurs appreciably and forms a non-negligible amount of hydroxide ion at equilibrium. In other words, the acetate ion does hydrolyze in water, producing OH– and thus yielding a basic aqueous solution.
While the above discussion compared a strong acid and a weak acid, we can extend the same arguments to a strong base and a weak base, and come to the following conclusions about ion hydrolysis:
- Conjugate bases of strong acids do NOT hydrolyze.
- Conjugate bases of weak acids DO hydrolyze.
- Conjugate acids of strong bases do NOT hydrolyze.
- Conjugate acids of weak bases DO hydrolyze.
Rules for Hydrolysis
Armed with the above understanding of the relative strength of conjugate acid-base pairs, we can now return to our discussion of salts (XY) and the 4 possible scenarios for ion hydrolysis. To determine the effect of a salt on solution pH, we must follow these steps:
- Determine the cation and anion produced when the salt is dissolved.
- Determine if the cation hydrolyzes or not. The cation X+ will hydrolyze in two situations:
- It is the conjugate acid of a weak base, and will generate H3O+ directly via the donation of a proton to water.
- It is a small, highly charged, metal cation, and will generate H3O+ indirectly via a different mechanism (note: this situation is not as common and is discussed in further detail at the end of this section).
3. Determine if the anion hydrolyzes or not. The anion Y– will hydrolyze if it is the conjugate base of a weak acid, and will generate OH– directly via the acceptance of a proton from water.
4. If both hydrolyze, perform additional analysis to find the overall effect on pH.
Hydrolysis of Salts: Qualitative Prediction of pH Changes
Let’s look at several examples and apply the above procedure to determine the acid-base character of different salts.
Example 1: NaCl
Sodium chloride dissolves in water to produce sodium cations and chloride anions:
NaCl (s) → Na+ (aq) + Cl– (aq)
The cation, Na+, does not hydrolyze: it is not the conjugate acid of a weak base (it cannot act as a proton donor!) and it is not a small, highly charged metal ion:
Na+ (aq) + H2O (l) → no hydrolysis
As seen already, the anion, Cl–, is the conjugate base of a strong acid (HCl), and so it also does not hydrolyze:
Cl– (aq) + H2O (l) → no hydrolysis
Therefore, since neither the cation nor the anion hydrolyze, neither H3O+ nor OH– is produced, and so overall, adding NaCl to an aqueous solution will have no effect on the pH. NaCl is thus called a NEUTRAL salt.
Example 2: NH4Br
Ammonium bromide dissolves in water to produce ammonium cations and bromide anions:
NH4Br (s) → NH4+ (aq) + Br– (aq)
The cation, NH4+, does hydrolyze: it is the conjugate acid of a weak base, NH3, and so will react with water in an equilibrium to produce some H3O+:
NH4+ (aq) + H2O (l) ⇌ NH3 (aq) + H3O+ (aq)
The anion, Br–, is the conjugate base of a strong acid (HBr), and so it does not hydrolyze:
Br– (aq) + H2O (l) → no hydrolysis
Therefore, since the cation hydrolyzes but the anion does not, overall, adding NH4Br to an aqueous solution will produce some H3O+ and the pH will decrease. NH4Br is thus called an ACIDIC salt.
Example 3: KCN
Potassium cyanide dissolves in water to produce potassium cations and cyanide anions:
KCN (s) → K+ (aq) + CN– (aq)
The cation, K+, does not hydrolyze: it is not the conjugate acid of a weak base, and it is not a small, highly charged metal ion:
K+ (aq) + H2O (l) → no hydrolysis
The anion, CN–, does hydrolyze: it is the conjugate base of a weak acid (HCN), and so will react with water in an equilibrium to produce some OH–:
CN– (aq) + H2O (aq) ⇆ HCN (aq) + OH– (aq)
Therefore, since the anion hydrolyzes but the cation does not, overall, adding KCN to an aqueous solution will produce some OH– and the pH will increase. KCN is thus called a BASIC salt.
Example 4: NH4CN
Ammonium cyanide dissolves in water to produce ammonium cations and cyanide anions:
NH4CN (s) → NH4+ (aq) + CN– (aq)
As seen above, the cation, NH4+, does hydrolyze:
NH4+ (aq) + H2O (l) ⇌ NH3 (aq) + H3O+ (aq)
Also seen above, the anion, CN–, also hydrolyzes:
CN– (aq) + H2O (aq) ⇆ HCN (aq) + OH– (aq)
Therefore, since BOTH the cation AND anion hydrolyze with water, we must now determine the extent of the two equilibria above. In other words, does the ammonium cation produce more H3O+ than the OH– produced by the cyanide anion or vice-versa? Which of these two equilibria is more product-favoured? To answer this, we simply need to calculate and compare the equilibrium constants of the two reactions above:
NH4+ (aq) + H2O (l) ⇌ NH3 (aq) + H3O+ (aq)
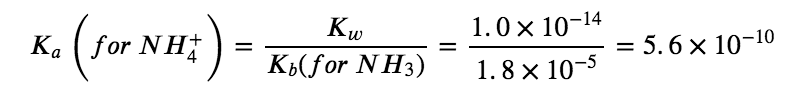
CN– (aq) + H2O (aq) ⇆ HCN (aq) + OH– (aq)
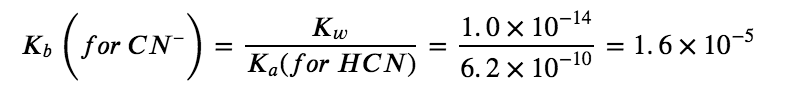
Comparing the two K values, we see that the Kb of the anion is greater than the Ka of the cation. The second equilibrium is more product-favoured than the first, and overall, more OH– than H3O+ is produced in solution as a result of the hydrolysis of both ions. Therefore, adding NH4CN to an aqueous solution will cause the pH to increase and it is thus classified as a BASIC salt.
Example 5.5.2 – Qualitative Prediction of pH Changes
Predict whether aqueous solutions of the following salts are acidic, basic, or neutral:
(a) KBr
(b) NaHCO3
(c) NH4Cl
(d) Na2HPO4
(e) NH4F
Solution
Consider each of the ions separately in terms of its effect on the pH of the solution, as shown here:
(a) The K+ cation and the Br− anion do not hydrolyze, since they are the cation of a strong base (KOH) and the anion of a strong acid (HBr), respectively. The solution is neutral.
(b) The Na+ cation does not hydrolyze, and will not affect the pH of the solution, while the HCO3− anion is amphiprotic. The Ka of HCO3− is 4.7 × 10−11, and its Kb is:
1.0×10-14/4.3×10-7=2.3×10-8
Since Kb >> Ka, this species is better at accepting protons than donating protons. Therefore, the bicarbonate anion will behave as a base and the solution is basic.
(c) The NH4+ ion is acidic and the Cl− ion does not hydrolyze. The solution will be acidic.
(d) The Na+ cation does not hydrolyze, and will not affect the pH of the solution, while the HPO42− anion is amphiprotic. The Ka of HPO42− is 4.2 × 10−13, and its Kb is:
1.0×10-14/6.2×10-8=1.6×10-7
Because Kb >> Ka, the solution is basic.
(e) The NH4+ ion is listed as being acidic, and the F− ion is listed as a base, so we must directly compare the Ka and the Kb of the two ions. Ka of NH4+ is 5.6 × 10−10, which seems very small, yet the Kb of F− is 1.4 × 10−11, so the solution is acidic, since Ka > Kb.
Check Your Learning 5.5.2 – Qualitative Prediction of pH Changes
Determine whether aqueous solutions of the following salts are acidic, basic, or neutral:
(a) K2CO3
(b) CaCl2
(c) KH2PO4
(d) (NH4)2CO3
(e) Mg(NO3)2
Answer
(a) basic; (b) neutral; (c) acidic; (d) basic; (e) neutral
Hydrolysis of Salts: Quantitative Prediction of pH Changes
The examples above provide an excellent illustration of how we can quickly determine the acid-base properties of ionic salts in aqueous solutions. But what if we need to calculate the exact effect on a solution’s pH? Below is an example to show how we can combine the described procedure with the equilibrium problem-solving steps used previously to find the final pH of salt solutions.
Example 5.5.3 – Quantitative Prediction of pH Changes
Aniline is an amine that is used to manufacture dyes. It is isolated as aniline hydrochloride, C6H5NH3Cl, a salt prepared by the reaction of the weak base aniline and hydrochloric acid. What is the pH of a 0.233 M solution of aniline hydrochloride?
Solution
Aniline hydrochloride dissolves in water to produce C6H5NH3+ cations and chloride anions:
C6H5NH3Cl (s) → C6H5NH3+ (aq) + Cl– (aq)
We know the anion, Cl–, does not hydrolyze:
Cl– (aq) + H2O (l) → no hydrolysis
However, the cation, C6H5NH3+, is the conjugate acid of a weak base, C6H5NH2 , and so it does hydrolyze:
C6H5NH3+ (aq) + H2O (l) ⇌ H3O+ (aq) + C6H5NH2 (aq)
Thus, following our previous procedure, at this point we predict an ACIDIC pH for this salt. However, to find the exact pH of the 0.233 M solution, our next step in this example is to determine Ka for the C6H5NH3+ ion. The value of Ka for this acid is not listed in Appendix H, but we can determine it from the value of Kb for aniline, C6H5NH2, which is given as 4.3 × 10−10 (Appendix I):
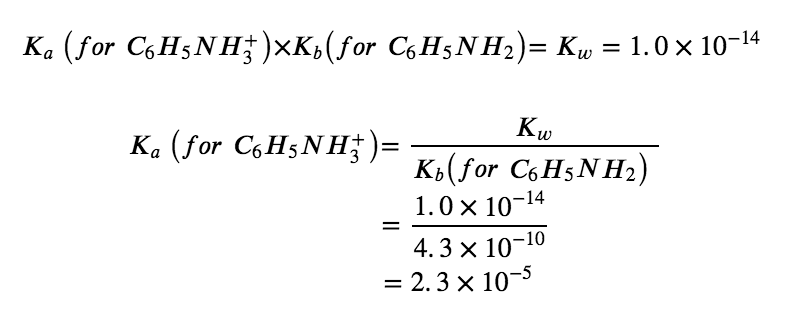
Now we have the ionization constant and the initial concentration of the weak acid, all the information necessary to determine the equilibrium concentration of H3O+, and the pH:
With these steps we find [H3O+] = 2.3 × 10−3 M and pH = 2.64, matching with the acidic pH we predicted qualitatively at the beginning. You should perform the calculations yourself to confirm these values.
Check Your Learning 5.5.3 – Quantitative Prediction of pH Changes
You are given a 0.100 M solution of ammonium nitrate, NH4NO3.
(a) Predict qualitatively: is this a neutral, acidic, or basic salt?
(b) Use the data in Appendix I to determine Ka for the ammonium ion.
(c) What is the hydronium ion concentration of the solution?
(d) What is the pH of the solution?
Answer
Acidic; (b) Ka (for NH4+) = 5.6 × 10−10; (c) [H3O+] = 7.5 × 10−6 M; (d) pH = 5.13
Example 5.5.4 – Quantitative Prediction of pH Changes
What is the pH of a 0.100 M solution of calcium hypochlorite, Ca(OCl)2?
Solution
Calcium hypochlorite dissolves in water to produce calcium cations and hypochlorite anions (note the stoichiometry):
Ca(OCl)2 (s) → Ca2+ (aq) + 2 OCl– (aq)
The cation, Ca2+, does not hydrolyze: it is not the conjugate acid of a weak base, and it is not a small, highly charged metal ion:
Ca2+ (aq) + H2O (l) → no hydrolysis
The anion, OCl–, does hydrolyze: it is the conjugate base of a weak acid (HOCl), and so will react with water in an equilibrium to produce some OH–:
OCl– (aq) + H2O (aq) ⇆ HOCl (aq) + OH– (aq)
Therefore, at this point, we predict a BASIC pH for the solution. The next step is to find the Kb for the OCl– ion using the Ka for HOCl from Appendix H:
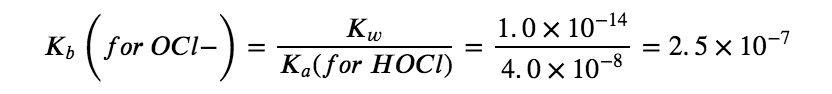
Combining this value along with the initial concentration of hypochlorite anion ([OCl–]i = 0.200 M, due to stoichiometry) we can use an ICE table to find that, at equilibrium, [OH–] = 2.2 x 10–4 M and pH = 10.35, matching our original prediction.
Check Your Learning 5.5.4 – Quantitative Prediction of pH Changes
What is the pH of a 0.083 M solution of LiCN?
Answer
11.06
The Ionization of Hydrated Metal Ions
As mentioned earlier in this Section, certain metal ions do hydrolyze, but via an indirect, alternate mechanism (i.e. not direct proton transfer to water molecules). If we measure the pH of the solutions of a variety of metal ions we will find that occasionally, these ions act as weak acids when in solution. The aluminum ion is an example. When aluminum nitrate dissolves in water, the aluminum ion reacts with water to give a hydrated aluminum ion, Al(H2O)63+, dissolved in bulk water. What this means is that the aluminum ion has the strongest interactions with the six closest water molecules (the so-called first solvation shell), even though it does interact with the other water molecules surrounding this Al(H2O)63+ cluster as well:
Al(NO3)3 (s) + 6 H2O (l) ⇌ Al(H3O+)63+ (aq) + 3 NO3– (aq)
Note: we frequently see the formula of this ion written simply as “Al3+(aq)”, without explicitly noting that six water molecules are bonded to the aluminum ion (similar to how, in older textbooks, the formula of the hydronium ion, H3O+, was simplified to H+(aq)).
Hydrated metal ions can behave as Brønsted-Lowry acids via an indirect proton transfer with water. Clearly, the aluminum atom itself cannot donate a proton – doing so would change the identity of the nucleus, transforming it into magnesium! Therefore, to act as Brønsted-Lowry acid, instead one of the bound water molecules acts as the proton donor to another external water molecule, becoming an OH– group and generating one equivalent of H3O+ in the process. This is depicted in Figure 5.5.3 below – note that the charge on the complex ion has reduced from +3 to +2, due to the loss of one positive charge.
Al(H2O)63+(aq)+H2O(l)⇌ H3O+(aq)+Al(H2O)5(OH)2+(aq) Ka=1.4×10-5
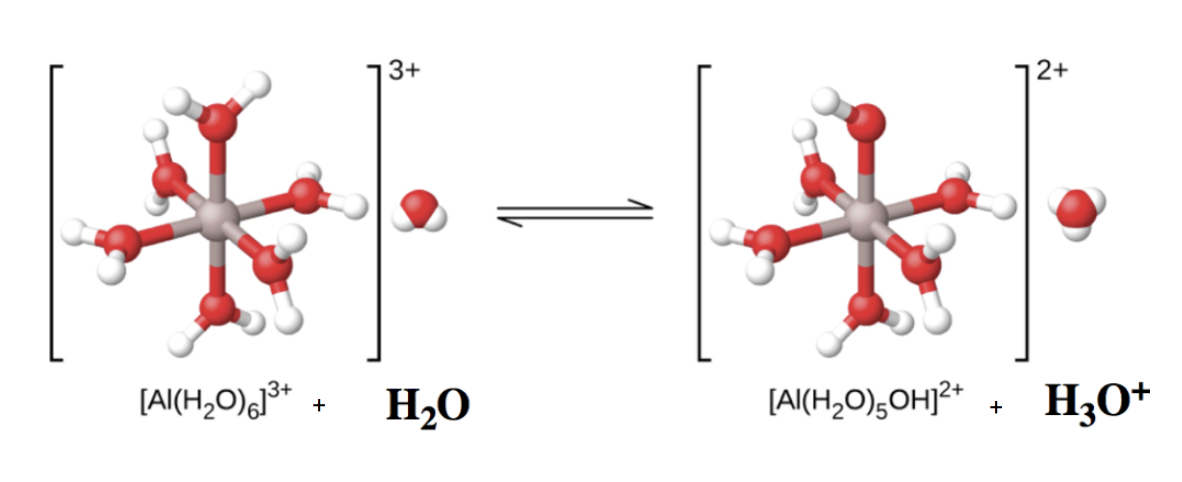
Figure 5.5.3. When an aluminum ion reacts with water, the hydrated aluminum ion becomes a weak acid. Note the top H2O ligand changes to an OH–. Additionally, the overall charge on the complex has decreased.
So, what other metal cations are capable of displaying similar acid behaviour? It is difficult to know for certain if a metal cation is acidic or not without experimental evidence. However, in general, a good rule of thumb is that metal cations with high charge densities tend to be significantly acidic. As the name implies, the charge density of an ion is the ratio of the charge of the ion to the volume of the ion, Equation 5.5.1:
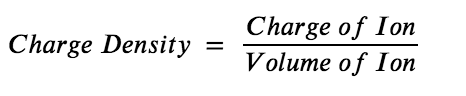
Equation 5.5.1. Charge Density.
Metal cations with high charges (e.g. ≥ +2) and small ionic radii will overall have high charge densities, and thus often display acidic character. Additional examples of acidic hydrated metal ions are:
Fe(H2O)63+ (aq) + H2O (l) ⇌ H3O+ (aq) + Fe(H2O)5(OH)2+ (aq) pKa = 2.74
Cu(H2O)62+ (aq) + H2O (l) ⇌ H3O+ (aq) + Cu(H2O)5(OH)+ (aq) pKa = ~6.3
Zn(H2O)42+ (aq) + H2O (l) ⇌ H3O+ (aq) + Zn(H2O)5(OH)+ (aq) pKa = 9.6
To further illustrate the acidity of these hydrated metal ions, the pH value of a 0.10 M solution of each are the following:
Fe(H2O)63+(aq) pH = 1.89
Cu(H2O)62+(aq) pH = 3.66
Zn(H2O)42+(aq) pH = 5.30
|
Note to CHM1311 Students:
So, ask your professor if you will be solving problems involving acidic metal ions, and if necessary, a table of relevant Ka values will be provided to you. |
Example 5.5.5 – Hydrolysis of [Al(H2O)6]3+
Calculate the pH of a 0.10 M solution of aluminum chloride, which dissolves completely to give the hydrated aluminum ion [Al(H2O)6]3+ in solution.
Solution
In spite of the unusual appearance of the acid, this is a typical acid ionization problem.
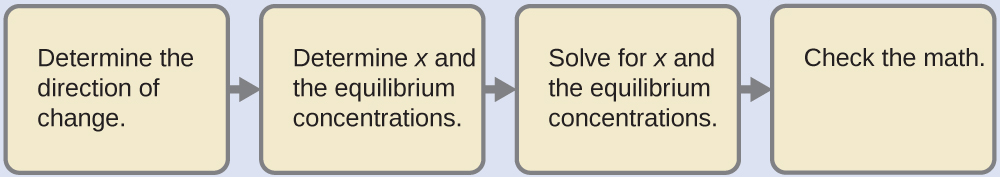
Determine the direction of change. The equation for the reaction and Ka are:
Al(H2O)63+(aq) + H2O (l) ⇌ H3O+ (aq) + Al(H2O)5(OH)2+(aq) Ka = 1.4 x 10-5
The reaction shifts to the right to reach equilibrium.
Determine x and equilibrium concentrations. Use the table:
Al(H2O)6 + H2O ⇌ H3O+ + Al(H2O)5(OH)
| Al(H2O)6 | H2O | H3O+ | Al(H2O)5(OH) | |
| Initial Concentration (M) | 0.10 | / | ~0 | 0 |
| Change (M) | – x | / | x | x |
| Equilibrium concentration (M) | 0.10 – x | / | x | x |
Solve for x and the equilibrium concentrations. Substituting the expressions for the equilibrium concentrations into the equation for the ionization constant yields:
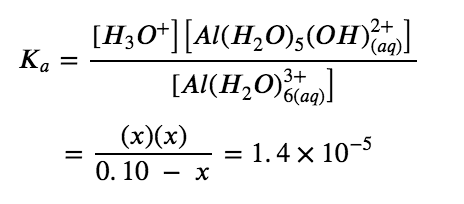
Solving this equation gives:
x=1.2×10-3 M
From this we find:
[H3O+]= 0+x=1.2×10-3 M
pH = -log[H3O+]= 2.92 (an acidic solution)
Check the work. The arithmetic checks; when 1.2 × 10−3 M is substituted for x, the result = Ka.
Check Your Learning 5.5.5 – Hydrolysis of [Al(H2O)6]3+
What is [Al(H2O)5(OH)2+] in a 0.15 M solution of Al(NO3)3 that also contains enough of the strong acid HNO3 to bring [H3O+] to 0.10 M?
Answer
2.1 × 10−5 M
Questions
★ Questions
- Determine whether aqueous solutions of the following salts are acidic, basic, or neutral:
(a) Al(NO3)3
(b) RbI
(c) KHCO2
(d) CH3NH3Br
2. Determine whether aqueous solutions of the following salts are acidic, basic, or neutral:
(a) FeCl3
(b) K2CO3
(c) NH4Br
(d) KClO4
★★ Questions
3. Novocaine, C13H21O2N2Cl, is the salt of the base procaine and hydrochloric acid. The ionization constant for procaine is 7 ×10−6. Is a solution of novocaine acidic or basic? What are [H3O+], [OH−], and pH of a 2.0% solution by mass of novocaine, assuming that the density of the solution is 1.0 g/mL.
Answers
- (a) acidic, (b) neutral, (c) basic, (d) acidic
- (a) acidic; (b) basic; (c) acidic; (d) neutral
- Acidic, [H3O+] = 3.2 x 10−5 M, [OH−] = 3.1 x 10−10 M, pH = 4.50
Reaction of an acid and a base to produce water and a salt

 P
P