8.2 – Atomic Spectra
Another paradox within the classical electromagnetic theory that scientists in the late nineteenth century struggled with concerned the light emitted from atoms and molecules. When solids, liquids, or condensed gases are heated sufficiently, they radiate some of the excess energy as light. Photons produced in this manner have a range of energies, and thereby produce a continuous spectrum in which an unbroken series of wavelengths is present. Most of the light generated from stars (including our sun) is produced in this fashion. You can see all the visible wavelengths of light present in sunlight by using a prism to separate them. As can be seen in Figure 8.1.8 in the previous topic, sunlight also contains UV light (shorter wavelengths) and IR light (longer wavelengths) that can be detected using instruments but that are invisible to the human eye. Incandescent (glowing) solids such as tungsten filaments in incandescent lights also give off light that contains all wavelengths of visible light. These continuous spectra can often be approximated by blackbody radiation curves at some appropriate temperature, such as those shown in Figure 8.1.9 in the previous section.
In contrast to continuous spectra, light can also occur as discrete or line spectra having very narrow line widths interspersed throughout the spectral regions such as those shown in Figure 8.2.2. Exciting a gas at low partial pressure using an electrical current, or heating it, will produce line spectra. Fluorescent light bulbs and neon signs operate in this way (Figure 8.2.1.). Each element displays its own characteristic set of lines, as do molecules, although their spectra are generally much more complicated.

Figure 8.2.1. Neon signs operate by exciting a gas at low partial pressure using an electrical current. This sign shows the elaborate artistic effects that can be achieved. (credit: Dave Shaver)
Each emission line consists of a single wavelength of light, which implies that the light emitted by a gas consists of a set of discrete energies. For example, when an electric discharge passes through a tube containing hydrogen gas at low pressure, the H2 molecules are broken apart into separate H atoms and we see a blue-pink color. Passing the light through a prism produces a line spectrum, indicating that this light is composed of photons of four visible wavelengths, as shown in Figure 8.2.2.
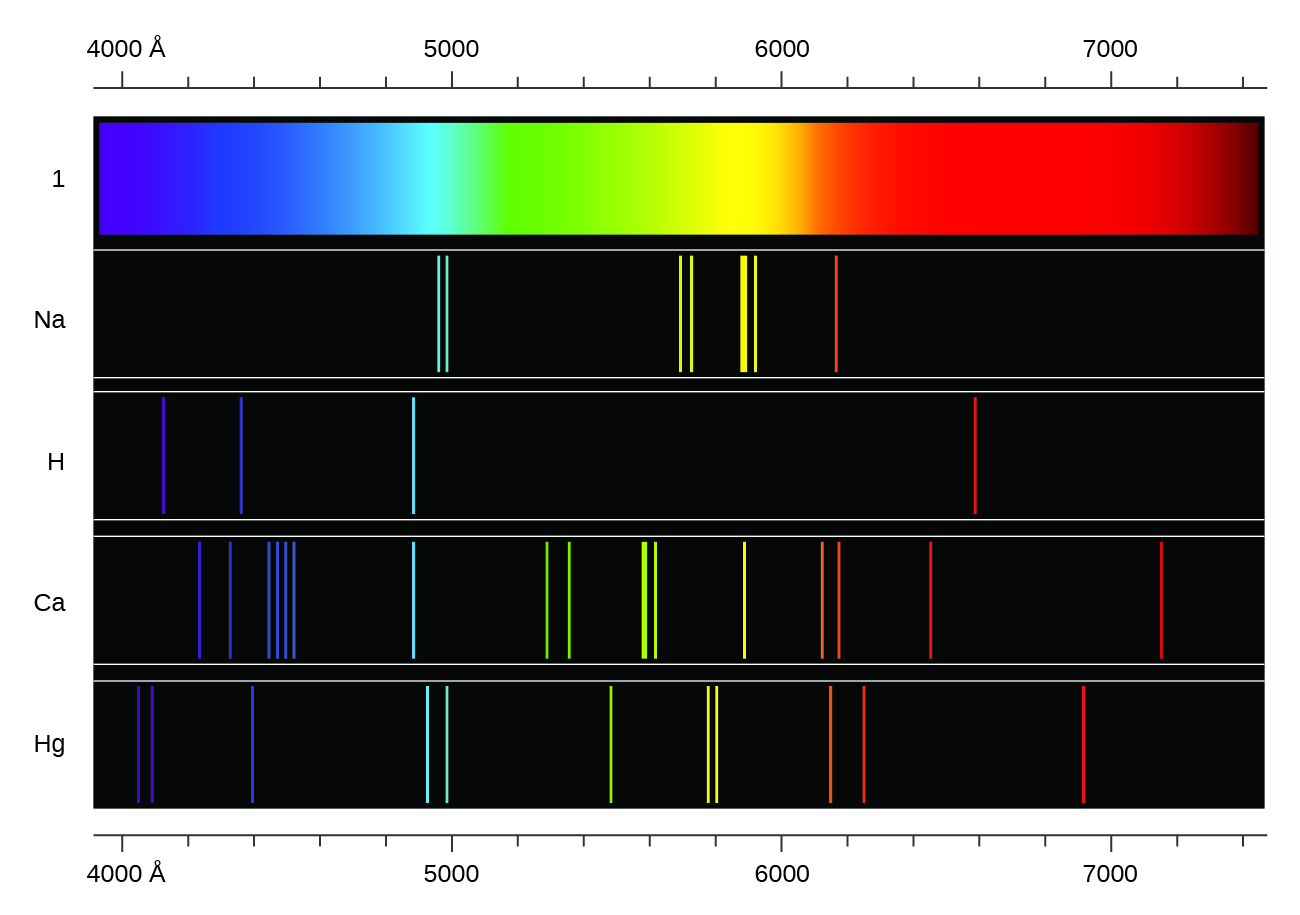
Figure 8.2.2. Compare the two types of emission spectra: continuous spectrum of white light (top) and the line spectra of the light from excited sodium, hydrogen, calcium, and mercury atoms.
The origin of discrete spectra in atoms and molecules was extremely puzzling to scientists in the late nineteenth century, since according to classical electromagnetic theory, only continuous spectra should be observed. Even more puzzling, in 1885, Johann Balmer was able to derive an empirical equation that related the four visible wavelengths of light emitted by hydrogen atoms to whole integers. That equation is the following one, in which k is a constant:
Other discrete lines for the hydrogen atom were subsequently found in the UV and IR regions. Johannes Rydberg generalized Balmer’s work and developed an empirical formula that predicted all of hydrogen’s emission lines, not just those restricted to the visible range, where, n1and n2 are integers, n1 < n2, and R∞ is the Rydberg constant (1.097 × 107 m−1).
Equation 8.2.1 Rydberg Equation
Even in the late nineteenth century, spectroscopy was a very precise science, and so the wavelengths of hydrogen were measured to very high accuracy, which implied that the Rydberg constant could be determined very precisely as well. That such a simple formula as the Rydberg formula could account for such precise measurements seemed astounding at the time, but it was the eventual explanation for emission spectra by Neils Bohr in 1913 that ultimately convinced scientists to abandon classical physics and spurred the development of modern quantum mechanics.
The Bohr Model
Following the work of Ernest Rutherford and his colleagues in the early twentieth century, the picture of atoms consisting of tiny dense nuclei surrounded by lighter and even tinier electrons continually moving about the nucleus was well established. This picture was called the planetary model, since it pictured the atom as a miniature “solar system” with the electrons orbiting the nucleus like planets orbiting the sun. The simplest atom is hydrogen, consisting of a single proton in the nucleus about which a single electron moves. The electrostatic force attracting the electron to the proton depends only on the distance between the two particles. The electrostatic force has the same form as the gravitational force between two mass particles except that the electrostatic force depends on the magnitudes of the charges on the particles (+1 for the proton and −1 for the electron) instead of the magnitudes of the particle masses that govern the gravitational force. Since forces can be derived from potentials, it is convenient to work with potentials instead, since they are forms of energy. The electrostatic potential is also called the Coulomb potential. Because the electrostatic potential has the same form as the gravitational potential, according to classical mechanics, the equations of motion should be similar, with the electron moving around the nucleus in circular or elliptical orbits (hence the label “planetary” model of the atom). Potentials of the form V(r) that depend only on the radial distance r are known as central potentials. Central potentials have spherical symmetry, and so rather than specifying the position of the electron in the usual Cartesian coordinates (x, y, z), it is more convenient to use polar spherical coordinates centered at the nucleus, consisting of a linear coordinate r and two angular coordinates, usually specified by the Greek letters theta (θ) and phi (Φ). These coordinates are similar to the ones used in GPS devices and most smartphones that track positions on our (nearly) spherical earth, with the two angular coordinates specified by the latitude and longitude, and the linear coordinate specified by sea-level elevation. Because of the spherical symmetry of central potentials, the energy and angular momentum of the classical hydrogen atom are constants, and the orbits are constrained to lie in a plane like the planets orbiting the sun. This classical mechanics description of the atom is incomplete, however, since an electron moving in an elliptical orbit would be accelerating (by changing direction) and, according to classical electromagnetism, it should continuously emit electromagnetic radiation. This loss in orbital energy should result in the electron’s orbit getting continually smaller until it spirals into the nucleus, implying that atoms are inherently unstable.
In 1913, Niels Bohr attempted to resolve the atomic paradox by ignoring classical electromagnetism’s prediction that the orbiting electron in hydrogen would continuously emit light. Instead, he incorporated into the classical mechanics description of the atom Planck’s ideas of quantization and Einstein’s finding that light consists of photons whose energy is proportional to their frequency. Bohr assumed that the electron orbiting the nucleus would not normally emit any radiation (the stationary state hypothesis), but it would emit or absorb a photon if it moved to a different orbit. The energy absorbed or emitted would reflect differences in the orbital energies according to this equation:
Equation 8.2.2 Orbital Energy Difference
In this equation, h is Planck’s constant and Ei and Ef are the initial and final orbital energies, respectively. The absolute value of the energy difference is used, since frequencies and wavelengths are always positive. Instead of allowing for continuous values for the angular momentum, energy, and orbit radius, Bohr assumed that only discrete values for these could occur (actually, quantizing any one of these would imply that the other two are also quantized). Bohr’s expression for the quantized energies is:
In this expression, k is a constant comprising fundamental constants such as the electron mass and charge and Planck’s constant. Inserting the expression for the orbit energies into the equation for ΔE gives
Or
which is identical to the Rydberg equation for
R∞ = khc
. When Bohr calculated his theoretical value for the Rydberg constant, R∞, and compared it with the experimentally accepted value, he got excellent agreement. Since the Rydberg constant was one of the most precisely measured constants at that time, this level of agreement was astonishing and meant that Bohr’s model was taken seriously, despite the many assumptions that Bohr needed to derive it.
The lowest few energy levels are shown in Figure 8.2.3. One of the fundamental laws of physics is that matter is most stable with the lowest possible energy. Thus, the electron in a hydrogen atom usually moves in the n = 1 orbit, the orbit in which it has the lowest energy. When the electron is in this lowest energy orbit, the atom is said to be in its ground electronic state (or simply ground state). If the atom receives energy from an outside source, it is possible for the electron to move to an orbit with a higher n value and the atom is now in an excited electronic state (or simply an excited state) with a higher energy.
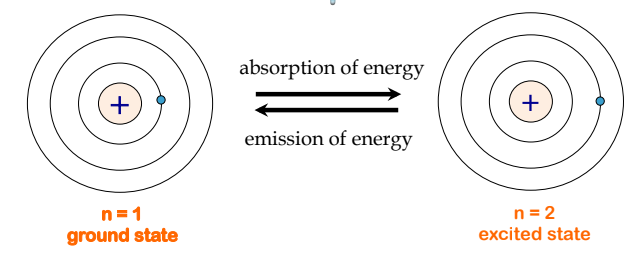
Figure 8.2.3. On the left, a hydrogen atom is shown in the lowest energy state, a.k.a. the ground state, where the electron is in the n = 1 orbit. When the atom absorbs energy, the electron is promoted to a higher orbit, resulting in a less stable, higher energy state shown on the right, known as an excited state. To return to the ground state, the atom must emit some energy.
When an electron transitions from an excited state (higher energy orbit) to a less excited state, or ground state, the difference in energy is emitted as a photon. Similarly, if a photon is absorbed by an atom, the energy of the photon moves an electron from a lower energy orbit up to a more excited one. We can relate the energy of electrons in atoms to what we learned previously about energy. The law of conservation of energy says that we can neither create nor destroy energy. Thus, if a certain amount of external energy is required to excite an electron from one energy level to another, that same amount of energy will be liberated when the electron returns to its initial state (Figure 8.2.2.). In effect, an atom can “store” energy by using it to promote an electron to a state with a higher energy and release it when the electron returns to a lower state. The energy can be released as one quantum of energy, as the electron returns to its ground state (say, from n = 5 to n = 1), or it can be released as two or more smaller quanta as the electron falls to an intermediate state, then to the ground state (say, from n = 5 to n = 4, emitting one quantum, then to n = 1, emitting a second quantum).
Since Bohr’s model involved only a single electron, it could also be applied to the single electron ions He+, Li2+, Be3+, and so forth, which differ from hydrogen only in their nuclear charges, and so one-electron atoms and ions are collectively referred to as hydrogen-like atoms. The energy expression for hydrogen-like atoms is a generalization of the hydrogen atom energy, in which Z is the nuclear charge (+1 for hydrogen, +2 for He, +3 for Li, and so on) and k has a value of 2.179 × 10–18 J.
The sizes of the circular orbits for hydrogen-like atoms are given in terms of their radii by the following expression, in which ɑ0 is a constant called the Bohr radius, with a value of 5.292 × 10−11 m:
Equation 8.2.3 Orbital Radius
The equation also shows us that as the electron’s energy increases (as n increases), the electron is found at greater distances from the nucleus. This is implied by the inverse dependence on r in the Coulomb potential, since, as the electron moves away from the nucleus, the electrostatic attraction between it and the nucleus decreases, and it is held less tightly in the atom. Note that as n gets larger and the orbits get larger, their energies get closer to zero, and so the limits n ⟶ ∞, and r ⟶ ∞ imply that E = 0 corresponds to the ionization limit where the electron is completely removed from the nucleus. Thus, for hydrogen in the ground state n = 1, the ionization energy would be:
With three extremely puzzling paradoxes now solved (blackbody radiation, the photoelectric effect, and the hydrogen atom), and all involving Planck’s constant in a fundamental manner, it became clear to most physicists at that time that the classical theories that worked so well in the macroscopic world were fundamentally flawed and could not be extended down into the microscopic domain of atoms and molecules. Unfortunately, despite Bohr’s remarkable achievement in deriving a theoretical expression for the Rydberg constant, he was unable to extend his theory to the next simplest atom, He, which only has two electrons. Bohr’s model was severely flawed, since it was still based on the classical mechanics notion of precise orbits, a concept that was later found to be untenable in the microscopic domain, when a proper model of quantum mechanics was developed to supersede classical mechanics.
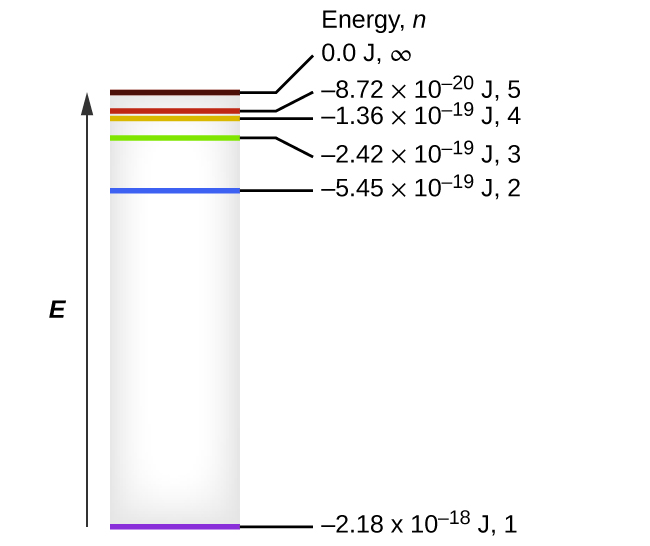
Figure 8.2.4. Quantum numbers and energy levels in a hydrogen atom. The more negative the calculated value, the lower the energy.
Example 8.2.1 – Calculating the Energy of an Electron in a Bohr Orbit
Early researchers were very excited when they were able to predict the energy of an electron at a particular distance from the nucleus in a hydrogen atom. If a spark promotes the electron in a hydrogen atom into an orbit with n = 3, what is the calculated energy, in joules, of the electron?
Solution
The energy of the electron is given by this equation:
The atomic number, Z, of hydrogen is 1; k = 2.179 × 10–18 J; and the electron is characterized by an n value of 3. Thus,
The electron in Figure 8.2.4. is promoted even further to an orbit with n = 6. What is its new energy?
Answer
−6.053 × 10–20 J
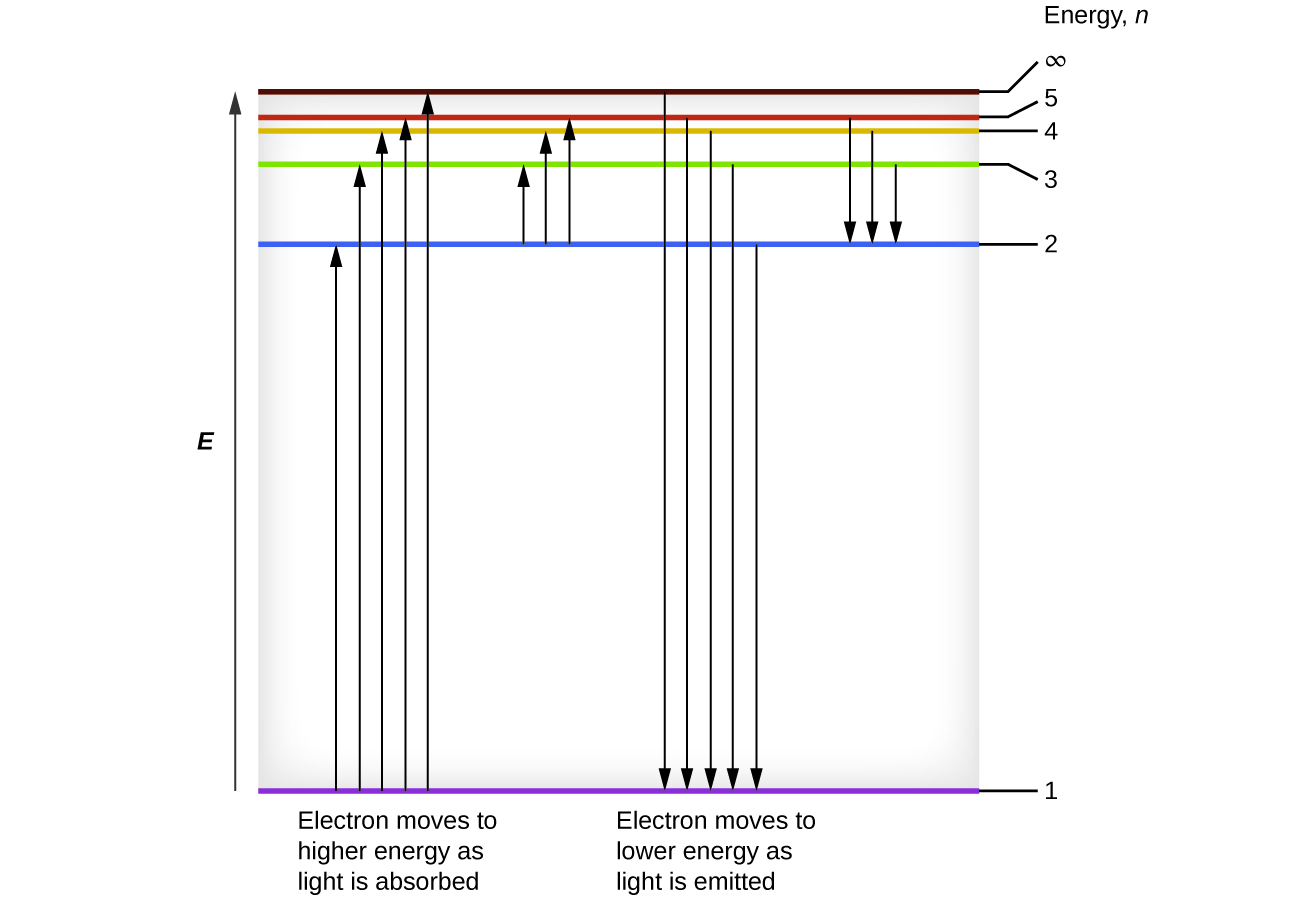
Figure 8.2.4. The horizontal lines show the relative energy of orbits in the Bohr model of the hydrogen atom, and the vertical arrows depict the energy of photons absorbed (left) or emitted (right) as electrons move between these orbits.
Example 8.2.2 – Calculating the Energy and Wavelength of Electron Transitions in a One–electron (Bohr) System
What is the energy (in joules) and the wavelength (in meters) of the line in the spectrum of hydrogen that represents the movement of an electron from Bohr orbit with n = 4 to the orbit with n = 6? In what part of the electromagnetic spectrum do we find this radiation?
Solution
In this case, the electron starts out with n = 4, so n1 = 4. It comes to rest in the n = 6 orbit, so n2 = 6. The difference in energy between the two states is given by this expression:
This energy difference is positive, indicating a photon enters the system (is absorbed) to excite the electron from the n = 4 orbit up to the n = 6 orbit. The wavelength of a photon with this energy is found by the expression
Rearrangement gives:
From Figure 8.2.1. of the previous topic, we can see that this wavelength is found in the infrared portion of the electromagnetic spectrum.
Check Your Learning 8.2.2 – Calculating the Energy and Wavelength of Electron Transitions in a One–electron (Bohr) System
What is the energy in joules and the wavelength in meters of the photon produced when an electron falls from the n = 5 to the n = 3 level in a He+ ion (Z = 2 for He+)?
Answer
6.198 × 10–19 J; 3.205 × 10−7 m
Bohr’s model of the hydrogen atom provides insight into the behavior of matter at the microscopic level, but it does not account for electron–electron interactions in atoms with more than one electron. It does introduce several important features of all models used to describe the distribution of electrons in an atom. These features include the following:
★ Questions
1. The light produced by a red neon sign is due to the emission of light by excited neon atoms. Qualitatively describe the spectrum produced by passing light from a neon lamp through a prism.
2. A bright violet line occurs at 435.8 nm in the emission spectrum of mercury vapor. What amount of energy, in joules, must be released by an electron in a mercury atom to produce a photon of this light?
3. Light with a wavelength of 614.5 nm looks orange.
a) What is the energy, in joules, per photon of this orange light?
b) What is the energy in eV (1 eV = 1.602 × 10−19 J)?
4. A photon of light produced by a surgical laser has an energy of 3.027 × 10−19 J.
a) Calculate the frequency and wavelength of the photon.
b) What is the total energy in 1 mole of photons?
c) What is the color of the emitted light?
5. The emission spectrum of cesium contains two lines whose frequencies are (a) 3.45 × 1014 Hz and (b) 6.53 × 1014 Hz.
a) What are the wavelengths and energies per photon of the two lines?
b) What color are the lines?
★★ Questions
6. RGB color television and computer displays use cathode ray tubes that produce colors by mixing red, green, and blue light. If we look at the screen with a magnifying glass, we can see individual dots turn on and off as the colors change.
a) Using a spectrum of visible light, determine the approximate wavelength of each of these colors.
b) What is the frequency and energy of a photon of each of these colors?
7.
a)What is the threshold frequency for sodium metal if a photon with frequency 6.66 × 1014 s−1 ejects an electron with 7.74 × 10−20 J kinetic energy?
b) Will the photoelectric effect be observed if sodium is exposed to orange light?
8. Heated lithium atoms emit photons of light with an energy of 2.961 × 10−19 J.
a) What is the total energy in 1 mole of these photons?
b) What is the color of the emitted light?
9. Photons of infrared radiation are responsible for much of the warmth we feel when holding our hands before a fire. These photons will also warm other objects. How many infrared photons with a wavelength of 1.5 × 10−6 m must be absorbed by the water to warm a cup of water (175 g) from 25.0 °C to 40 °C?
10. The eyes of certain reptiles pass a single visual signal to the brain when the visual receptors are struck by photons of a wavelength of 850 nm. If a total energy of 3.15 × 10−14 J is required to trip the signal, what is the minimum number of photons that must strike the receptor?
Answers
1. The spectrum consists of colored lines, at least one of which (probably the brightest) is red.
2. 4.56 x 10-19 J
3. a) 3.233 × 10−19 J; b) 2.018 eV
4. a) v = 4.568 × 1014 s; b) λ = 656.3 nm; c) Energy mol−1 = 1.823 × 105 J mol−1; red
5. a) λ = 8.69 × 10−7 m; E = 2.29 × 10−19 J; (b) λ = 4.59 × 10−7 m; E = 4.33 × 10−19 J; The color of (a) is red; (b) is blue.
6. Red: 660 nm; 4.54 × 1014 Hz; 3.01 × 10−19 J. Green: 520 nm; 5.77 × 1014 Hz; 3.82 × 10−19 J. Blue: 440 nm; 6.81 × 1014 Hz; 4.51 × 10−19 J. Somewhat different numbers are also possible.
7. a) 5.49 × 1014 s−1; b) no
8. a) Energy in 1 mole of photons: 178.3 kJ; b) color: red
9. 8.3 x 1022 photons
10. 1.3 x 105 photons
Electromagnetic radiation emitted at discrete wavelengths (i.e. contains only certain colours of light) by a specific atom (or atoms) in an excited state
Structural model in which an electron moves around the nucleus only in circular orbits, each with a specific allowed radius
State in which the electrons in an atom, ion, or molecule have the lowest energy possible
State in which the electrons in an atom, ion, or molecule have the lowest energy possible
State having an energy greater than the ground-state energy
State having an energy greater than the ground-state energy
Number having only specific allowed values and used to characterize the arrangement of electrons in an atom

