4.1 – Introduction to Chemical Equilibrium
A chemical reaction is usually written in a way that suggests it proceeds in one direction, the direction in which we read, but all chemical reactions are reversible, and both the forward and reverse reaction occur to one degree or another depending on conditions – this is the notion of chemical equilibrium which we will discuss here in this chapter.
Dynamic Equilibrium
In a chemical equilibrium, the forward and reverse reactions occur at equal rates, and the concentrations of products and reactants remain constant. If we run a reaction in a closed system so that the products cannot escape, we often find the reaction does not give a 100% yield of products. Instead, some reactants remain after the concentrations stop changing. At this point, when there is no further change in concentrations of reactants and products, we say the reaction is at equilibrium. A mixture of reactants and products is found at equilibrium.
For example, when we place a sample of dinitrogen tetroxide (N2O4, a colorless gas) in a glass tube, it forms nitrogen dioxide (NO2, a brown gas) by the following reaction:
N2O4 (g) ⇌ 2 NO2 (g)
The color becomes darker as N2O4 is converted to NO2. When the system reaches equilibrium, both N2O4 and NO2 are present (Figure 4.1.1).
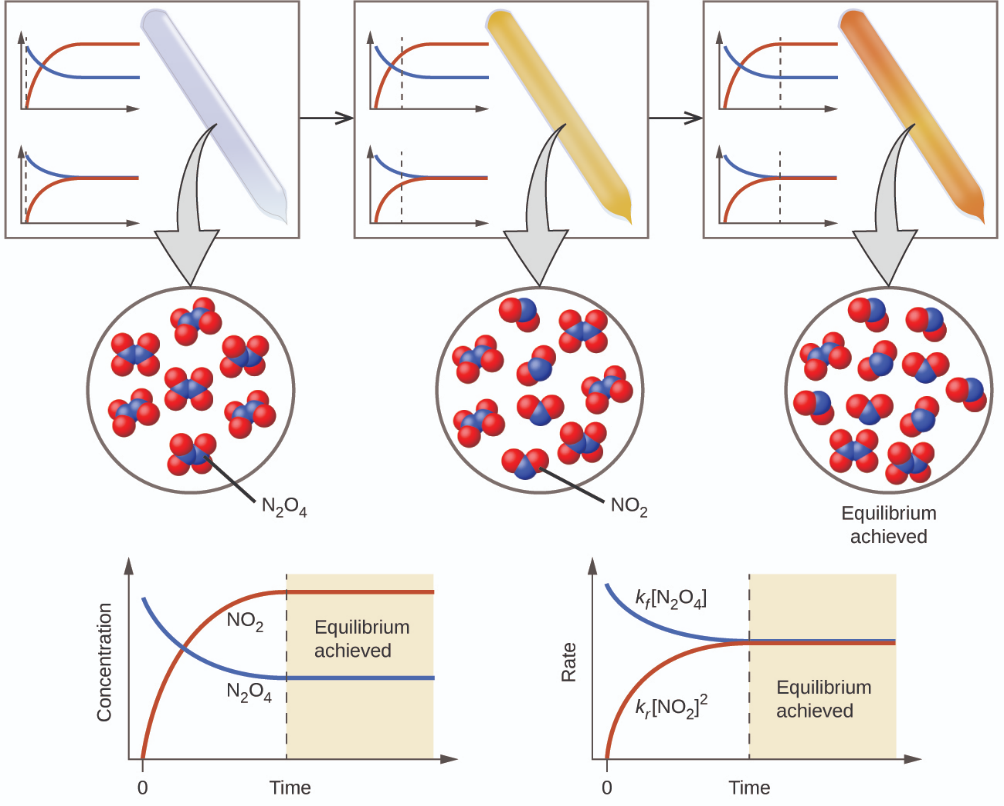
Figure 4.1.1. A mixture of NO2 and N2O4 moves toward equilibrium. Colorless N2O4 reacts to form brown NO2. As the reaction proceeds toward equilibrium, the color of the mixture darkens due to the increasing concentration of NO2. (Chem Libre, From: https://chem.libretexts.org/Bookshelves/General_Chemistry/Map%3A_General_Chemistry_(Petrucci_et_al.)/15%3A_Principles_of_Chemical_Equilibrium/15.1%3A_Dynamic_Equilibrium, Creative Commons)
The formation of NO2 from N2O4 is a reversible reaction, which is identified by the equilibrium arrows (⇌). All reactions are reversible, but many reactions, for all practical purposes, proceed in the forward direction until the reactants are exhausted and will reverse only under certain conditions. Such reactions are often depicted with a one-way arrow from reactants to products. Many other reactions, such as the formation of NO2 from N2O4, are reversible under more easily obtainable conditions and, therefore, are named as such. In a reversible reaction, the reactants can combine to form products and the products can react to form the reactants. Thus, not only can N2O4 decompose to form NO2, but the NO2 produced can react to form N2O4. As soon as the forward reaction produces any NO2, the reverse reaction begins and NO2 starts to react to form N2O4. At equilibrium, the concentrations of N2O4 and NO2 no longer change because the rate of formation of NO2 is exactly equal to the rate of consumption of NO2, and the rate of formation of N2O4 is exactly equal to the rate of consumption of N2O4. Hence we get to a key idea: Chemical equilibrium is a dynamic process. For example, if two jugglers are each tossing clubs at the other at the same rate at which each receives clubs from the other, the numbers of clubs each will have at any point in time will remain roughly constant, yet there is always a flux back and forth between them (Figure 4.1.2).

Figure 4.1.2. These jugglers provide an illustration of dynamic equilibrium. Each throws clubs to the other at the same rate at which he receives clubs from that person. Because clubs are thrown continuously in both directions, the number of clubs moving in each direction is constant, and the number of clubs each juggler has at a given time remains (roughly) constant.
In a chemical equilibrium, the forward and reverse reactions do not stop, rather they continue to occur at the same rate, leading to constant concentrations of the reactants and the products. Plots showing how the reaction rates and concentrations change with respect to time are shown in Figure 4.1.1.
We can detect a state of equilibrium because the concentrations of reactants and products do not appear to change. However, it is important that we verify that the absence of change is due to equilibrium and not to a reaction rate that is so slow that changes in concentration are difficult to detect.
We use a double arrow when writing an equation for a reversible reaction. Such a reaction may or may not be at equilibrium. For example, Figure 4.1.1 shows the reaction:
|
N2O4 (g) colorless
|
kf ⇌ kr
|
2 NO2 (g) red-brown
|
When we wish to speak about one particular component of a reversible reaction, we use a single arrow. For example, in the equilibrium shown in Figure 4.1.1, the rate of the forward reaction with rate constant kf
|
N2O4 (g) colorless
|
Kf →
|
2 NO2 (g) red-brown
|
is equal to the rate of the backward reaction with rate constant kr
|
N2O4 (g) colorless
|
Kr ←
|
2 NO2 (g) red-brown
|
We’ll talk more about rate constants in Chapter 7: Chemical Kinetics; for now, all you need to know is that each rate constant is unique to a particular reaction that helps mathematically define its rate of reaction.
|
Equilibrium & Soft Drinks |
|
The connection between chemistry and carbonated soft drinks goes back to 1767, when Joseph Priestley (1733–1804; mostly known today for his role in the discovery and identification of oxygen) discovered a method of infusing water with carbon dioxide to make carbonated water. In 1772, Priestly published a paper entitled “Impregnating Water with Fixed Air.” The paper describes dripping oil of vitriol (today we call this sulfuric acid, but what a great way to describe sulfuric acid: “oil of vitriol” literally means “liquid nastiness”) onto chalk (calcium carbonate). The resulting CO2 falls into the container of water beneath the vessel in which the initial reaction takes place; agitation helps the gaseous CO2 mix into the liquid water. H2SO4 (l) + CaCO3 (s) ⇌ CO2 (g) + H2O (l) + CaSO4 (aq) Carbon dioxide is slightly soluble in water. There is an equilibrium reaction that occurs as the carbon dioxide reacts with the water to form carbonic acid (H2CO3). Since carbonic acid is a weak acid, it can ionize into hydronium ions (H3O+) and hydrogen carbonate ions (HCO3−). CO2 (aq) + H2O (l) ⇌ H2CO3 (aq) H2O (l) + H2CO3 (aq) ⇌ H3O+ (aq) + HCO3– (aq) Today, CO2 can be pressurized into soft drinks, establishing the equilibrium shown above. Once you open the beverage container, however, a cascade of equilibrium shifts occurs. First, the CO2 gas in the air space on top of the bottle escapes, causing the equilibrium between gas-phase CO2 and dissolved or aqueous CO2 to shift, lowering the concentration of CO2 in the soft drink. Less CO2 dissolved in the liquid leads to carbonic acid decomposing to dissolved CO2 and H2O. The lowered carbonic acid concentration causes a shift of the final equilibrium. As long as the soft drink is in an open container, the CO2 bubbles up out of the beverage, releasing the gas into the air (Figure 4.1.3). With the lid off the bottle, the CO2 reactions are no longer at equilibrium and will continue until no more of the reactants remain. This results in a soft drink with a much lowered CO2 concentration, often referred to as “flat.”
Figure 4.1.3. When a soft drink is opened, several equilibrium shifts occur. (credit: modification of work by “D Coetzee”/Flickr) |
The equilibrium between N2O4 and NO2 consists of a chemical equilibrium – one which involves two or more substances through a chemical reaction. However, an equilibrium consisting of a single substance, known as a physical equilibrium, can be established for a physical change—like a liquid to gas transition—as well as for a chemical reaction. Let us consider the evaporation of bromine as a second example of a system at equilibrium.
Br2 (l) ⇌ Br2 (g)
Figure 4.1.4 shows a sample of liquid bromine at equilibrium with bromine vapour in a closed container. When we pour liquid bromine into an empty bottle in which there is no bromine vapour, some liquid evaporates, the amount of liquid decreases, and the amount of vapour increases. If we cap the bottle so no vapour escapes, the amount of liquid and vapour will eventually stop changing and an equilibrium between the liquid and the vapour will be established. If the bottle were not capped, the bromine vapour would escape and no equilibrium would be reached.

Figure 4.1.4. An equilibrium is pictured between liquid bromine, Br2 (l), the dark liquid, and bromine vapour, Br2 (g), the reddish-brown gas. Because the container is sealed, bromine vapour cannot escape and equilibrium is maintained. (credit: http://images-of-elements.com/bromine.php)
Deriving a Constant for Chemical Equilibria
Because an equilibrium state is achieved when the forward reaction rate equals the reverse reaction rate, under a given set of conditions there must be a relationship between the composition of the system at equilibrium and the kinetics of a reaction (represented by rate constants). Let’s continue using the example of the decomposition of N2O4 to NO2 to demonstrate this. Both the forward and reverse reactions for this system consist of a single elementary reaction, so the reaction rates are as follows:
forward rate = kf[N2O2]
and
reverse rate = kr[NO2]2
Note: You’ll learn all about rate expressions and expressing reaction rates in the chapter on kinetics, but for now, we’ll briefly explain these expressions. As previously mentioned, the rate constant, k, is unique to a particular reaction and helps mathematically define the rate of reaction. The concentration of reactant also determines the rate; the reason why NO2 is squared in the expression for the reverse rate is because 2 molecules of NO2 are required to form N2O4, as indicated in the chemical equilibrium equation. Note that the value of the exponent is based on the number of molecules of reactant only for elementary reactions; again don’t worry about understanding what this means right now, since it will be properly covered later on.
At equilibrium, the forward rate equals the reverse rate (definition of equilibrium):
kf[N2O2] = kr[NO2]2
so
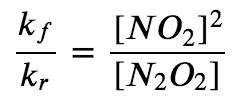
The ratio of the rate constants gives us a new constant, the equilibrium constant (K), which is defined as follows:
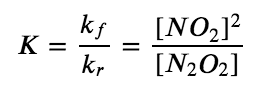
Hence there is a fundamental relationship between chemical kinetics and chemical equilibrium: under a given set of conditions, the composition of the equilibrium mixture is determined by the magnitudes of the rate constants for the forward and the reverse reactions. In other words, this equilibrium constant is equal to the rate constant for the forward reaction divided by the rate constant for the reverse reaction.
Measure of Reaction Extent
Because there is a direct relationship between the kinetics of a reaction and the equilibrium concentrations of products and reactants, when kf ≫ kr, K has a large value, and the concentration of products at equilibrium predominate. This corresponds to an essentially irreversible reaction. Conversely, when kf ≪ kr, K has a very small value, and the reaction produces almost no products as written. Systems for which kf ≈ kr have a value of K close to 1 and significant concentrations of both reactants and products at equilibrium.
Consider an equilibrium between substances A and B: A ⇌ B. The table below shows the percentage of moles of A and B that would be present at equilibrium for different values of K. At K values of 10-5 and 105, the mole percent of A and B are very nearly 100%, respectively. Hence, keep the following as a rule of thumb for this course: for K < 10-5, the reaction is said to be “reactant-favoured” and consists of mostly reactant(s) with minimal product(s), whereas for K > 105, the reaction practically proceeds to completion with little to no reactant(s) remaining.
Table 4.1.1 Relationship between the magnitude of K and mole percent of reactant and product.
|
A ⇌ B |
||
|
K |
mol% A |
mol% B |
|
100 000 |
0.001 |
99.999 |
|
10 000 |
0.01 |
99.99 |
|
1 000 |
0.10 |
99.90 |
|
100 |
0.99 |
99.01 |
|
10 |
9.09 |
90.91 |
|
1 |
50.00 |
50.00 |
|
0.1 |
90.91 |
9.09 |
|
0.01 |
99.01 |
0.99 |
|
0.001 |
99.90 |
0.10 |
|
0.0001 |
99.99 |
0.01 |
|
0.00001 |
99.999 |
0.001 |
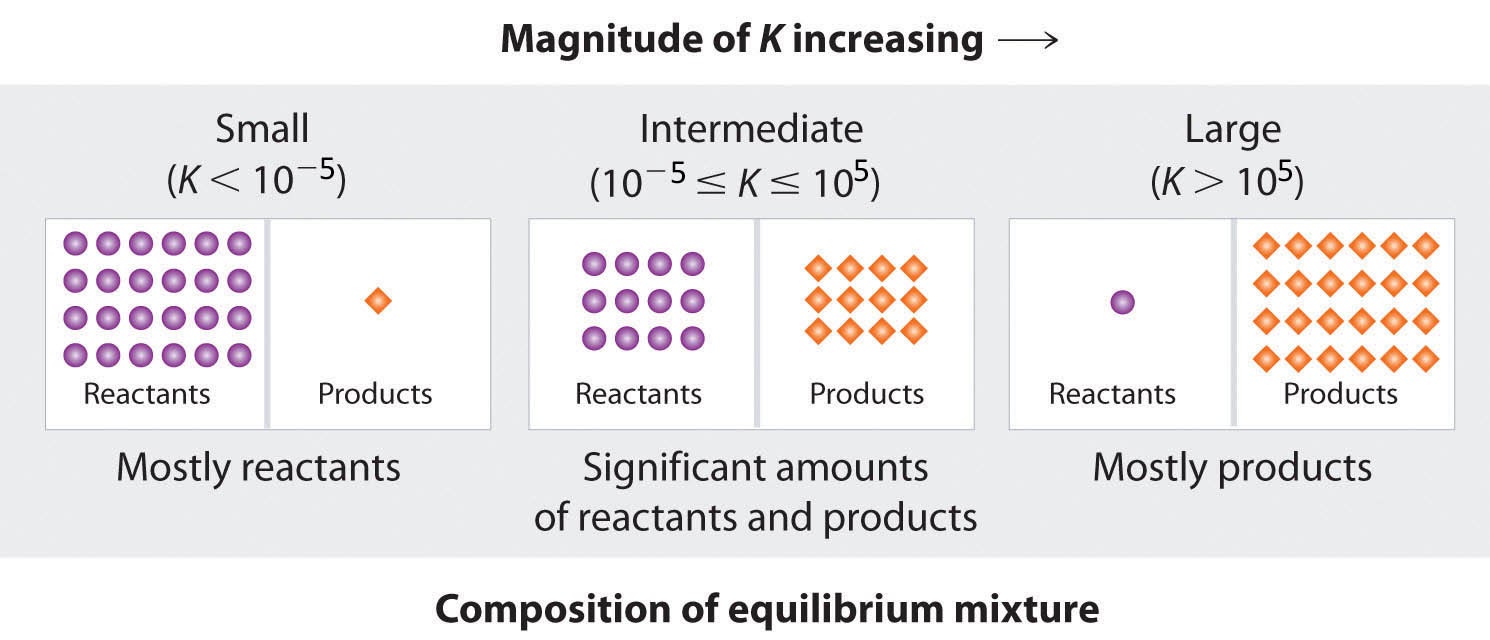
Figure 4.1.5. The larger the value of K, the farther the reaction proceeds to the right before equilibrium is reached, and the greater the ratio of products to reactants at equilibrium.
Let’s look at two examples of reactions to understand this:
H2(g) + Cl2(g) ⇌ 2 HCl(g) K = 1.6×1033 at 300K
F2(g) ⇌ 2 F(g) K = 7.4×10–13 at 500K
The reaction between H2 and Cl2 to produce HCl has an equilibrium constant of 1.6 × 1033 at 300 K. Because H2 is a good reductant and Cl2 is a good oxidant, the value of K is extremely large and the reaction proceeds essentially to completion. On the other hand, the reaction at 500 K between F2 and two F atoms in the gas phase has an equilibrium constant of 7.4 × 10-13. This K value is extremely small, indicating that the reactants don’t tend to form products readily – the formation and presence of reactants is heavily favoured.
|
When Should You Use a One-Sided Arrow? |
|
You may have noticed so far that in some reactions, equilibrium (two-direction) arrows are used, while in others only forward arrows are employed. The deciding factor on which arrow format is appropriate is the equilibrium constant (K) value. From Table 4.1.1, we see that if the value of K is 105, when equilibrium is reached, the system will contain about 0.001% of original reactant. This remainder is essentially negligible, and so we state that this product-favoured reaction therefore ‘goes to completion’, indicating ~100% expected formation of products. Thus, when encountering reactions with values of K ≥ 105, we tend to use one-sided, forward direction arrows only. In all other scenarios, the forward and reverse directions must be considered, and therefore it is more appropriate to use the two-directional equilibrium arrows. |
Example 4.1.1 – Composition of Reactants/Products at Equilibrium
Predict which systems at equilibrium will (a) contain essentially only products, (b) contain essentially only reactants, and (c) contain appreciable amounts of both products and reactants.
(a) H2 (g) + I2 (g) ⇌ 2 HI (g) K(700 K) = 54
(b) 2 CO2 (g) ⇌ 2 CO (g) + O2 (g) K(1200 K) = 3.1 × 10-18
(c) PCl5 (g) ⇌ PCl3 (g) + Cl2 (g) K(613 K) = 97
(d) 2 O3 (g) ⇌ 3 O2 (g) K(298 K) = 5.9 × 1055
Solution
(a) K is closer to 1 (10-5 < K < 105), so the equilibrium mixture will contain appreciable amounts of both products and reactants..
(b) K << 1 (K < 10-5), so the reactants have little tendency to form products under the conditions specified; thus, at equilibrium the system will contain essentially only reactants.
(c) K is closer to 1 (10-5 < K < 105), so the equilibrium mixture will contain appreciable amounts of both products and reactants.
(d) K >> 1 (K > 105), so at equilibrium it will consist of essentially only products.
Check Your Learning 4.1.1 – Composition of Reactants/Products at Equilibrium
Hydrogen and nitrogen react to form ammonia according to the following balanced chemical equation:
N2 (g) + 3 H2 (g) ⇌ 2 NH3 (g)
Values of the equilibrium constant at various temperatures were reported as
K25°C = 3.3 × 108
K177°C = 2.6 × 103
K327°C = 4.1
(a) At which temperature would you expect to find the highest proportion of H2 and N2 in the equilibrium mixture?
(b) Assuming that the reaction rates are fast enough so that equilibrium is reached quickly, at what temperature would you design a commercial reactor to operate to maximize the yield of ammonia?
Answer
(a) 327°C, where K is smallest; (b) 25°C, where K is largest
Questions
★ Questions
- What does it mean to describe a reaction as “reversible”?
2. When writing an equation, how is a reversible reaction distinguished from a non reversible reaction?
3. If a reaction is reversible, when can it be said to have reached equilibrium?
4. Is a system at equilibrium if the rate constants of the forward and reverse reactions are equal?
5. If the concentrations of products and reactants are equal, is the system at equilibrium?
Answers
- The reaction can proceed in both the forward and reverse directions.
2. Between the two types of reactions, they are distinguished by the arrows used in the equation. In a reversible reaction “⇌” is appropriate, whereas in a non- reversible reaction “→” is used.
3. When a system has reached equilibrium, no further changes in the reactant and product concentrations occur; the forward and reverse reactions continue to occur, but at equivalent rates.
4. Not necessarily; equivalent values of kf and kr (for the forward and reverse directions) yield an equilibrium constant value of 1 (since K = kf/kr). However, this does not mean that the system is at equilibrium. Only constant concentrations of the reactant and products mean the reactions are at equilibrium. This does not mean that their concentrations have to be equal, but in some cases they can be.
5. The concept of equilibrium does not imply equal concentrations, though it is possible.
State of a reversible reaction in which the forward and reverse processes occur at equal rates
Chemical reaction that can proceed in both the forward and reverse directions under given conditions


