3.1 – Introduction to Thermochemistry
Over 90% of the energy we use comes originally from the sun. Every day, the sun provides the earth with almost 10,000 times the amount of energy necessary to meet all of the world’s energy needs for that day. Our challenge is to find ways to convert and store incoming solar energy so that it can be used in reactions or chemical processes that are both convenient and non-polluting. Plants and many bacteria capture solar energy through photosynthesis. We release the energy stored in plants when we burn wood or plant products such as ethanol. We also use this energy to fuel our bodies by eating food that comes directly from plants or from animals that got their energy by eating plants. Burning coal and petroleum also releases stored solar energy: These fuels are fossilized plant and animal matter.
This chapter will introduce the basic ideas of an important area of science concerned with the amount of heat absorbed or released during chemical and physical changes—an area called thermochemistry. The concepts introduced in this chapter are widely used in almost all scientific and technical fields. Food scientists use them to determine the energy content of foods. Biologists study the energetics of living organisms, such as the metabolic combustion of sugar into carbon dioxide and water. The oil, gas, and transportation industries, renewable energy providers, and many others endeavour to find better methods to produce energy for our commercial and personal needs. Engineers strive to improve energy efficiency, find better ways to heat and cool our homes, refrigerate our food and drinks, and meet the energy and cooling needs of computers and electronics, among other applications. Understanding thermochemical principles are essential for chemists, physicists, biologists, geologists, every type of engineer, and just about anyone who studies or does any kind of science.
What is Energy?
Energy can be defined as the capacity to produce heat or do work. One type of work (W) is the process of causing matter to move against an opposing force. For example, we do work when we inflate a bicycle tire—we move matter (the air in the pump) against the opposing force of the air already in the tire.
Like matter, energy comes in different types. One scheme classifies energy into two types: potential energy, the energy an object has because of its relative position, composition, or condition, and kinetic energy, the energy that an object possesses because of its motion. Water at the top of a waterfall or dam has potential energy because of its position; when it flows downward through generators, it has kinetic energy that can be used to do work and produce electricity in a hydroelectric plant (Figure 3.1.1). A battery has potential energy because the chemicals within it can produce electricity that can do work.

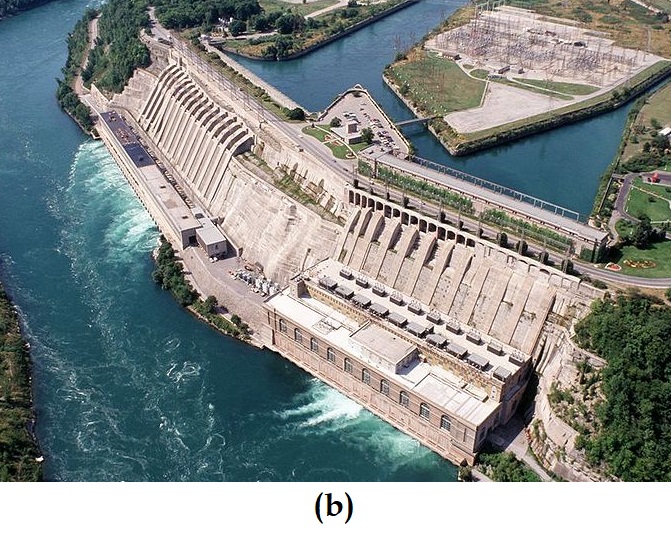
Figure 3.1.1. (a) Water that is higher in elevation, for example, at the top of Niagara Falls, has a higher potential energy than water at a lower elevation. As the water falls, some of its potential energy is converted into kinetic energy. (b) If the water flows through generators at the bottom of a dam, such as the Sir Adam Beck Hydroelectric Generating Stations in Niagara Falls, Ontario, shown here, its kinetic energy is converted into electrical energy. (credit a: Photo by Ivan Torres from Pexels ; credit b: Adam Beck Complex by Ontario Power Generation has been modified (cropped) and is used under a CC BY 2.0 license.)
Energy can be converted from one form into another, but all of the energy present before a change occurs always exists in some form after the change is completed. This observation is expressed in the law of conservation of energy: during a chemical or physical change, energy can be neither created nor destroyed, although it can be changed in form. (This is also one version of the first law of thermodynamics, as you will learn later.)
When one substance is converted into another, there is always an associated conversion of one form of energy into another. Heat is usually released or absorbed, but sometimes the conversion involves light, electrical energy, or some other form of energy. For example, chemical energy (a type of potential energy) is stored in sugar molecules like sucrose. When the sugar molecules react with O2 with the help of a reducing agent (e.g. potassium chlorate, KClO3), the chemical reaction causes the chemical energy stored in the molecular bonds to be released and converted into other forms of energy including heat (the combustion being an exothermic process) and light (see the box “Gummy Bear Combustion”).
|
Gummy Bear Combustion |
|
Check out this experimental demonstration video* where a gummy bear is combusted using molten potassium chlorate as a reducing agent. The potassium chlorate is added and heated until it becomes molten (observed at 1:07). Notice what happens when the green gummy bear is added to the molten potassium chlorate at around 1:18. * [C for Chemistry]. (2010, February 13). “Gummy Bear Experiment” with molten Potassium chlorate [Video file]. Retrieved from https://www.youtube.com/watch?v=7Xu2YZzufTM |
According to the law of conservation of matter (seen in an earlier chapter), there is no detectable change in the total amount of matter during a chemical change. When chemical reactions occur, the energy changes are relatively modest and the mass changes are too small to measure, so the laws of conservation of matter and energy hold well. However, in nuclear reactions, the energy changes are much larger (by factors of a million or so), the mass changes are measurable, and matter-energy conversions are significant. To encompass both chemical and nuclear changes, we combine these laws into one statement: The total quantity of matter and energy in the universe is fixed.
Units of Energy
Historically, energy was measured in units of calories (cal). A calorie is the amount of energy required to raise one gram of water by 1°C (1 kelvin). However, this quantity depends on the atmospheric pressure and the starting temperature of the water. The ease of measurement of energy changes in calories has meant that the calorie is still frequently used. The Calorie (with a capital C), or nutritional calorie, commonly used in quantifying food energy content, is a kilocalorie (1 kcal), or 1000 cal (see the box “Measuring Nutritional Calories if you’re interested). The SI unit of heat, work, and energy is the joule. A joule (J) is defined as the amount of energy used when a force of 1 newton moves an object 1 meter. It is named in honour of the English physicist James Prescott Joule. One joule is equivalent to 1 kg m2/s2, which is also called 1 newton–meter. A kilojoule (kJ) is 1000 joules. To standardize its definition, 1 calorie has been set to equal 4.184 joules.
1 calorie = 4.184 J
A great deal of the problems you’ll be dealing with in this unit will express energy in joules, however, it’s very important to remember how to convert between joules and calories. Despite the joule predominating over time as the official SI unit for energy, the calorie unit is still extensively used in the industrial sector today and a lot of tabulated data for compounds still expresses values in terms of calories. So be aware of what units of energy you’re working with and have the ability to perform unit conversions when necessary.
|
Measuring Nutritional Calories |
|
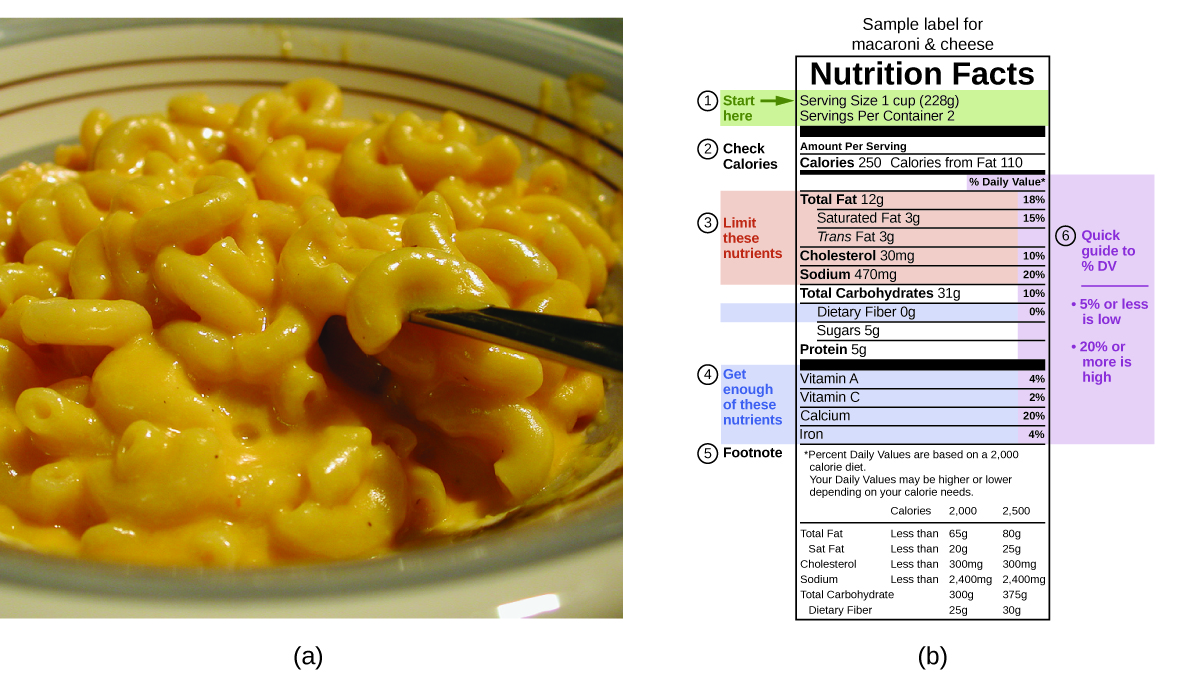
In your day-to-day life, you may be more familiar with energy being given in Calories, or nutritional calories, which are used to quantify the amount of energy in foods. One calorie (cal) = exactly 4.184 joules, and one Calorie (note the capitalization) = 1000 cal, or 1 kcal. (This is approximately the amount of energy needed to heat 1 kg of water by 1°C.) The macronutrients in food are proteins, carbohydrates, and fats or oils. Proteins provide about 4 Calories per gram, carbohydrates also provide about 4 Calories per gram, and fats and oils provide about 9 Calories/g. Nutritional labels on food packages show the caloric content of one serving of the food, as well as the breakdown into Calories from each of the three macronutrients (Figure 3.1. 2).
Figure 3.1.2 (a) Macaroni and cheese contain energy in the form of the macronutrients in the food. (b) The food’s nutritional information is shown on the package label. In Canada (and the US), the energy content is given in Calories; the rest of the world usually uses kilojoules. (credit a: modification of work by “Rex Roof”/Flickr) For the example shown in (b), the total energy per 228-g portion is calculated by: (5 g protein × 4 Calories/g) + (31 g carb × 4 Calories/g) + (12 g fat × 9 Calories/g) = 252 Calories So, you can use food labels to count your Calories. But where do the values come from? And how accurate are they? The caloric content of foods can be determined by using bomb calorimetry; that is, by burning the food and measuring the energy it contains. A sample of food is weighed, mixed in a blender, freeze-dried, ground into powder, and formed into a pellet. The pellet is burned inside a bomb calorimeter, and the measured temperature change is converted into energy per gram of food (bomb calorimetry is covered in much more detail in the section “Calorimetry”). Today, the caloric content on food labels is derived using a method called the Atwater system that uses the average caloric content of the different chemical constituents of food, protein, carbohydrate, and fats. The average amounts are those given in the equation and are derived from the various results given by bomb calorimetry of whole foods. The carbohydrate amount is discounted a certain amount for the fibre content, which is an indigestible carbohydrate. To determine the energy content of food, the quantities of carbohydrate, protein, and fat are each multiplied by the average Calories per gram for each and the products summed to obtain the total energy. |
Energy in the Universe
Before we can begin to study and understand the flow of energy in the context of a chemical reaction, we need to distinguish between a system, the small, well-defined part of the universe being examined (such as a chemical reaction), and its surroundings, which is the environment immediately around it (such as the reaction vessel, the lab bench etc.). The universe is defined as everything, both the surroundings and the system (Figure 3.1.3). In the discussion that follows, the mixture of chemical substances that undergoes a reaction is always the system, and the flow of heat can be from the system to the surroundings or vice versa.
Figure 3.1.3. The system is that part of the universe we are interested in studying, such as a chemical reaction inside a flask. The surroundings are the rest of the universe, including the container in which the reaction is carried out.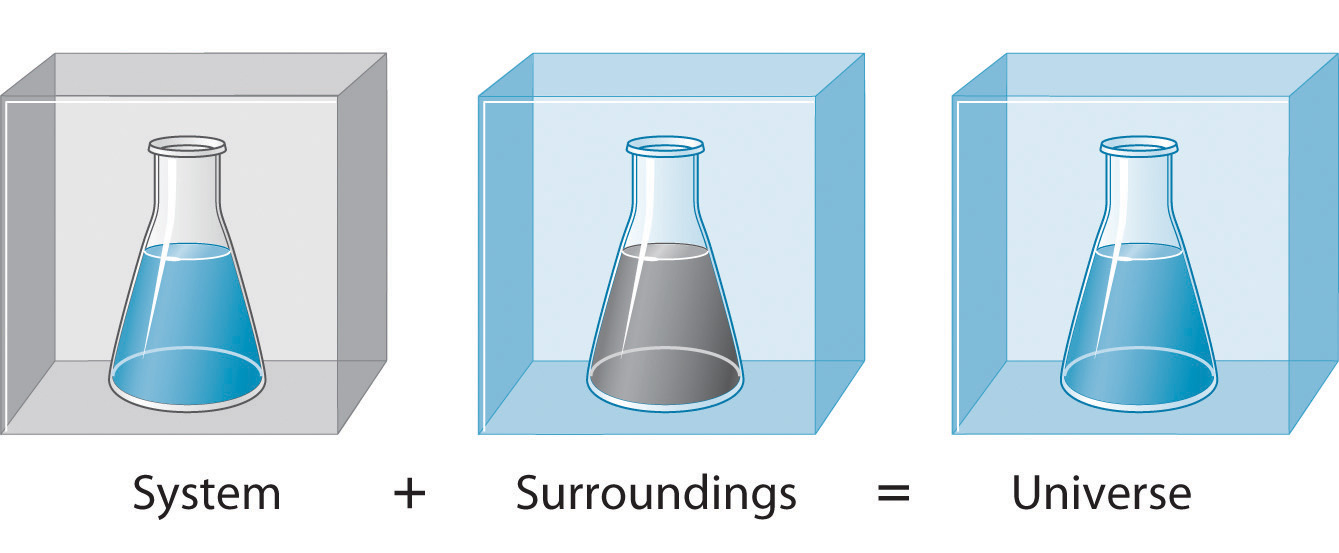
Three kinds of systems are important in chemistry. An open system can exchange both matter and energy with its surroundings. A pot of boiling water is an open system because a burner supplies energy in the form of heat, and matter in the form of water vapour is lost as the water boils. A closed system can exchange energy but not matter with its surroundings. The sealed pouch of a ready-made dinner that is dropped into a pot of boiling water is a closed system because thermal energy is transferred to the system from the boiling water but no matter is exchanged (unless the pouch leaks, in which case it is no longer a closed system). An isolated system exchanges neither energy nor matter with the surroundings. Energy is always exchanged between a system and its surroundings, although this process may take place very slowly. A truly isolated system does not actually exist. An insulated thermos containing hot coffee approximates an isolated system, but eventually, the coffee cools as heat is transferred to the surroundings. In all cases, the amount of heat lost by a system is equal to the amount of heat gained by its surroundings and vice versa. In other words, just like it was said previously in our introduction to energy: the total energy of a system plus its surroundings (i.e. the universe) is constant/fixed, which must be true if energy is conserved.
Questions
★ Questions
Newton’s cradle, a desk ornament that consists of 5 metal spheres suspended on wires that can freely swing into each other, demonstrates conservation of energy by showing that energy can be transferred through each sphere to make one several centimetres away move. This should be able to repeat forever, but it does stop eventually. Is this in violation of the law of conservation of energy? If not, where has the energy gone?
The idea of perpetual motion machines (theoretical machines that continue to do work forever by recycling all of its energy) has been fascinating humans for centuries. Why is this not possible in practice?
A cup of strawberries contains 53 Cal (nutritional calories). How much energy is this in Joules?
You’ve just baked a cake in your oven. Identify the system, surroundings, and the universe.
Answers
This does not violate the law of conservation of energy because the energy is transferred to different kinds rather than remaining in the form the system strictly needs. In this case, it can transform into sound, thermal, and more. This prevents it from going on forever.
The perpetual motion machine is a similar case to question 1. It is impossible to isolate a system to ensure that the transfer of energies remain what they are intended to be. The machine will eventually stop as it involves energies transforming into others that are not needed for this system, such as sound, thermal, etc.
1.3 × 104 J
System = cake, surroundings = oven, universe = cake and oven
Study of measuring the amount of heat absorbed or released during a chemical reaction or a physical change
Capacity to supply heat or do work
Energy transfer due to changes in external, macroscopic variables such as pressure and volume; or causing matter to move against an opposing force
Energy of a particle or system of particles derived from relative position, composition, or condition
Energy of a moving body, in joules, equal to 1/2 mvE2 (where m = mass and v = velocity)
Unit of heat or other energy; the amount of energy required to raise 1 gram of water by 1 degree Celsius; 1 cal is defined as 4.184 J
SI unit of energy; 1 J = 1 kg m2 s-2 and 4.184 J = 1 cal
Portion of matter undergoing a chemical or physical change being studied
All matter other than the system being studied
System that exchanges both matter and energy with its surroundings
System that can exchange energy but not matter with its surroundings
System that exchanges neither energy nor matter with the surroundings