2.2 – Gases and the Periodic Table
The geometric structure and the physical and chemical properties of atoms, ions, and molecules usually do not depend on their physical state; the individual water molecules in ice, liquid water, and steam, for example, are all identical. In contrast, the macroscopic properties of a substance depend strongly on its physical state, which is determined by intermolecular forces and conditions such as temperature and pressure.
Figure 2.2.1 shows the locations in the periodic table of those elements that are commonly found in the gaseous, liquid, and solid states. Except for hydrogen, the elements that occur naturally as gases are on the right side of the periodic table. Of these, all the noble gases (group 18) are monatomic gases, whereas the other gaseous elements are diatomic molecules (H2, N2, O2, F2, and Cl2). Oxygen can also form a second allotrope, the highly reactive triatomic molecule ozone (O3), which is also a gas. In contrast, bromine (as Br2) and mercury (Hg) are liquids under normal conditions (25 °C and 1.0 atm, commonly referred to as “room temperature and pressure”). Gallium (Ga), which melts at only 29.76 °C, can be converted to a liquid simply by holding a container of it in your hand or keeping it in a non-air-conditioned room on a hot summer day. The rest of the elements are all solids under normal conditions.
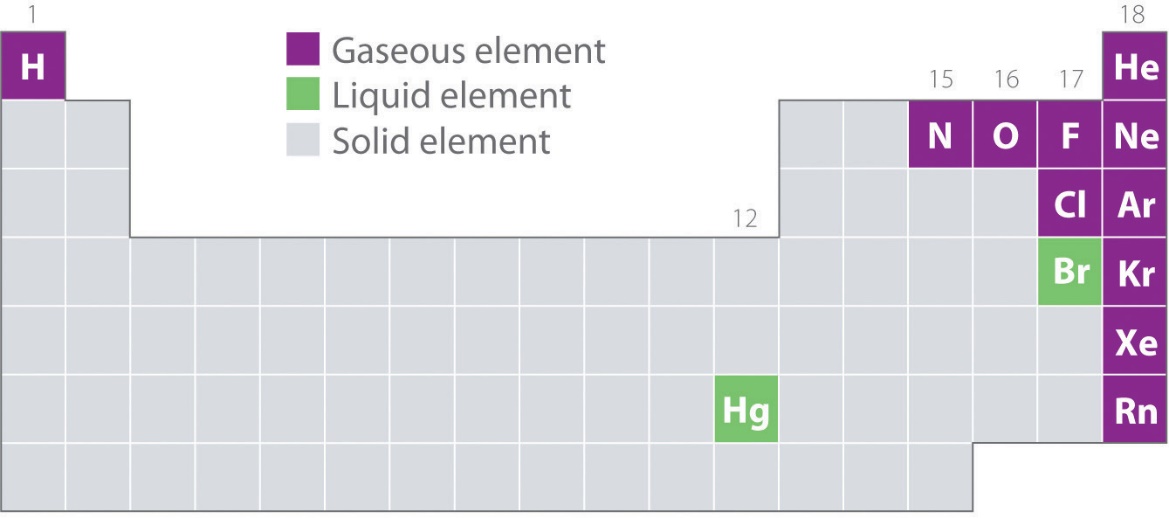
Figure 2.2.1. Elements that occur naturally as gases, liquids, and solids at 25 °C and 1 atm. The noble gases and mercury occur as monatomic species, whereas all other gases and bromine are diatomic molecules.
All of the gaseous elements (other than the monatomic noble gases) are molecules. Within the same group (1, 15, 16 and 17), the lightest elements are gases. All gaseous substances are characterized by weak interactions between the constituent molecules or atoms. When referring to nongaseous elements, we use the term vapour. The term vapour refers to the gaseous form of a substance that is normally a liquid or a solid under standard conditions. Thus, nitrogen (N2) and oxygen (O2) are referred to as gases, but gaseous water in the atmosphere is called water vapour.
Questions
★ Questions
1. Neon, a gas at room temperature, condenses into a liquid at 25 K. Which chemical properties of neon are affected by this phase change?
2. Like oxygen, carbon also exists in multiple naturally-occurring allotropes. Are any of them in the gaseous state under normal conditions?
Answers
1. None, the inherent chemical properties of an element remain unchanged when there is a change in phase.
2. No. The three major allotropes of carbon – graphite, diamond, and fullerenes – are all solids under normal conditions.
Material in the gas phase due to evaporation

