6.2 – Buffer Solutions
A mixture of a weak acid and its conjugate base (or a mixture of a weak base and its conjugate acid) is called a buffer solution, or simply a buffer. Buffer solutions resist a change in pH when small amounts of a strong acid or a strong base are added (Figure 6.2.1). A solution of acetic acid and sodium acetate (CH3COOH + CH3COONa) is an example of a buffer that consists of a weak acid and its conjugate base, whereas a solution of ammonia and ammonium chloride (NH3 + NH4Cl) is an example of a buffer that consists of a weak base and its conjugate acid.

Figure 6.2.1. (a) The unbuffered solution on the left and the buffered solution on the right have the same pH (pH 8); they are basic, showing the yellow colour of the indicator methyl orange at this pH. (b) After the addition of 1 mL of a 0.01 M HCl solution, the buffered solution has not detectably changed its pH but the unbuffered solution has become acidic, as indicated by the change in colour of the methyl orange, which turns red at a pH of about 4. (credit: modification of work by Mark Ott)
How Buffers Work
Buffers are made from mixing a weak acid with its conjugate base or a weak base with its conjugate acid. It is vital that the acid and base form a conjugate pair; this way, the two species cannot neutralize one another. For example, if a buffer is formed from a weak acid HA and its conjugate base A–, any proton transfer that occurs yields products that are identical to the reactants, a process known as an identity reaction:
HA + A– → A– + HA
Instead, the acid and base conjugate pair function to neutralize external sources of base and acid, respectively, in the following reactions:
HA + OH– → A– + H2O
A– + H3O– → HA + H2O
We will return to these neutralization reactions later, but first let us examine the differences between acidic and basic buffers.
Acidic Buffers: Aqueous Mixtures of HA + A–
If we mix together acetic acid and sodium acetate in water, the resulting aqueous solution has a pH < 7. It is acidic because the Ka of acetic acid (1.8 x 10–5) is greater than the Kb of the conjugate base acetate (5.6 x 10–10):
CH3COOH + H2O ⇌ CH3COO– + H3O+ Ka = 1.8 x 10–5
CH3COO– + H2O ⇌ CH3COOH + OH– Kb = 5.6 x 10–10
Comparing the K values, the first equilibrium lies further towards products than the second, thus there is more hydronium ion than hydroxide ion in the mixture, producing an overall pH for the mixture that is acidic.
This solution is a buffer because it contains the conjugate pair of a weak acid and its conjugate base, HA and A–, and both species are present in significant concentrations. This acts to keep the hydronium ion concentration (the pH) almost constant even after the addition of a strong acid or a strong base. The conjugate acid/base pair is able to neutralize the strong acid or base added to the solution.
For example, if an external source of acid increases the amount of H3O+, the buffer counteracts this addition:
CH3COO– (aq) + H3O+ (aq) → CH3COOH (aq) + H2O (l)
In this reaction, the conjugate base, CH3COO–, will neutralize the added acid, H3O+. This reaction goes to completion, because the reaction of CH3COO– with H3O+ has an equilibrium constant that is the inverse of the Ka for CH3COOH: 1/Ka = 1/(1.8 x 10-5) = 5.5 x 104, indicating a heavily product-favoured reaction. So long as there is significantly more CH3COO– than H3O+, the externally added H3O+ will be consumed, increasing the concentration of CH3COOH and decreasing the concentration of CH3COO– . But, since these two species are both present in large concentrations, the ratio of acid-to-conjugate base is nearly constant and there will be hardly any change in the amount of H3O+ present once equilibrium is re-established.
If a strong base were added such as sodium hydroxide (NaOH):
CH3COOH + OH– (aq) + OH– (aq) ⇌ CH3COO– (aq) + H2O (l)
In this reaction, the conjugate acid, CH3COOH, will neutralize added amounts of base, OH–, slightly increasing the concentration of CH3COO– in the solution and decreasing the amount of CH3COOH. Again, since most of the OH– is neutralized, little pH change will occur.
Basic Buffers: aqueous mixtures of B + HB+
A mixture of ammonia and ammonium chloride is basic because the Kb for ammonia is greater than the Ka for the ammonium ion. It is a buffer because it also contains the conjugate acid of the weak base. If we add a base (hydroxide ions), ammonium ions in the buffer react with the hydroxide ions to form ammonia and water and reduce the hydroxide ion concentration almost to its original value:
NH4+ (aq) + OH– (aq) → NH3 (aq) + H2O (l)
If we add an acid (hydronium ions), ammonia molecules in the buffer mixture react with the hydronium ions to form ammonium ions and reduce the hydronium ion concentration almost to its original value:
H3O+ (aq) + NH3 (aq) → NH4+ (aq) + H2O (l)
The three parts of the following example illustrate the change in pH that accompanies the addition of base to a buffered solution of a weak acid and to an unbuffered solution of a strong acid.
Example 6.2.1 – pH Changes in Buffered and Unbuffered Solutions
Acetate buffers are used in biochemical studies of enzymes and other chemical components of cells to prevent pH changes that might change the biochemical activity of these compounds.
(a) Calculate the pH of an acetate buffer that is a mixture of 0.10 M acetic acid and 0.10 M sodium acetate.
(b) Calculate the pH after 1.0 mL of 0.10 M NaOH is added to 100 mL of this buffer, giving a solution with a total volume of 101 mL.
(c) Calculate the pH after 1.0 mL of 0.10 M NaOH is added to an 100 mL of an unbuffered solution of 1.8 × 10−5 M HCl.
Solution
(a) To determine the pH of the buffer solution we use a typical equilibrium calculation (as illustrated in earlier Examples):
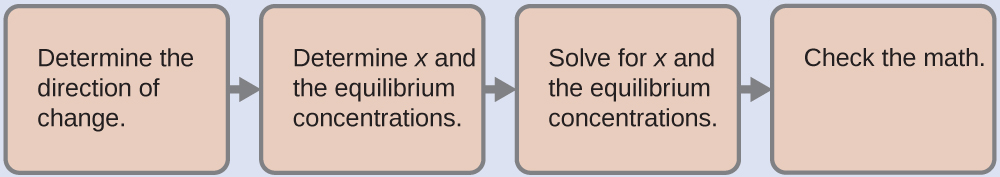
Determine the direction of change. The equilibrium in a mixture of H3O+, CH3CO2−, and CH3CO2H is:
CH3CO2H (aq) + H2O (l) ⇌ H3O+ (aq) + CH3CO2– (aq)
The equilibrium constant for CH3CO2H is not given, so we look it up in Appendix H: Ka = 1.8 × 10−5. With [CH3CO2H]i = [CH3CO2−]i= 0.10 M and [H3O+]i = ~0 M, the reaction shifts to the right to form H3O+.
Determine x and equilibrium concentrations. A table of changes and concentrations follows:
CH3CO2H + H2O ⇌ H3O+ + CH3CO2–
|
|
CH3CO2H |
H2O |
H3O+ |
CH3CO2– |
|
Initial Concentration (M) |
0.10 |
/ |
~0 |
0.10 |
|
Change (M) |
-x |
/ |
x |
x |
|
Equilibrium concentration (M) |
0.10-x |
/ |
x |
0.10 + x |
Solve for x and the equilibrium concentrations. We find:
x = 1.8×10-5 M
and
[H3O+] = 0+x = 1.8×10-5 M
Thus:
Check the work. If we calculate all calculated equilibrium concentrations, we find that the equilibrium value of the reaction coefficient, Q = Ka.
Note: Notice that when [HA] = [A-], pH = pKa. Qualitatively, one would expect a decrease in pH as the [HA] increases since this increases the [H3O+]. Inversely, one would expect an increase in pH as the [A–] increases. Since A– is the conjugate base of HA (see equation below), by increasing the concentration of A–, the equilibrium will favour HA (based on Le Chatelier’s Principle) thereby decreasing the [H3O+] in solution and therefore increasing the pH. Later in this chapter, the Henderson-Hasselbach equation will be introduced and will mathematically demonstrate why pH = pKa when [HA] = [A-].
(b) Since we are adding base, it will be neutralized by the acid of the buffer, acetic acid. First, we calculate the concentrations of an intermediate mixture resulting from the complete reaction between the acid in the buffer and the added base. Then we determine the concentrations of the mixture at the new equilibrium:
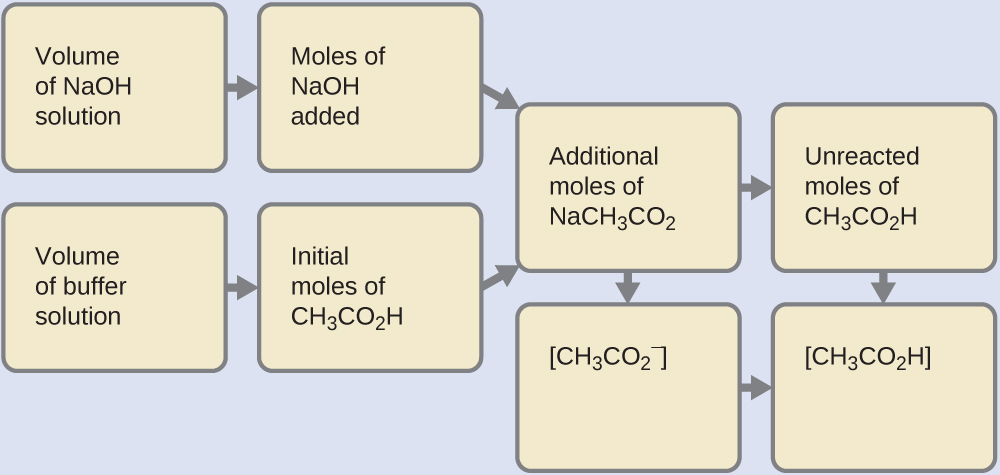
Determine the moles of NaOH. One milliliter (0.0010 L) of 0.10 M NaOH contains:
0.0010 L×(0.10 mol NaOH/1 L) = 1.0×10–4 mol NaOH
Determine the moles of CH2CO2H. Before reaction, 0.100 L of the buffer solution contains:
0.100 L ×(0.100 mol CH3CO2H/1L) = 1.00×10-2 mol CH3CO2H
Solve for the amount of NaCH3CO2 produced. The 1.0×10−4 mol of NaOH neutralizes 1.0×10−4 mol of CH3CO2H, leaving:
(1.0×10-2)-(0.01×10-2) = 0.99×10-2 mol CH3CO2H
and producing 1.0 × 10−4 mol of NaCH3CO2. This makes a total of:
(1.0×10-2)+(0.01×10-2) = 1.01×10-2 mol NaCH3CO2
Find the molarity of the products. After reaction, CH3CO2H and NaCH3CO2 are contained in 101 mL of the intermediate solution, so:
Now we calculate the pH after the intermediate solution, which is 0.098 M in CH3CO2H and 0.100 M in NaCH3CO2, comes to equilibrium. The calculation is very similar to that in part (a) of this example:
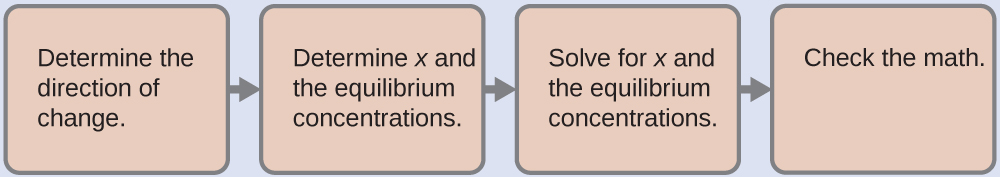
This series of calculations gives a pH = 4.75. Thus the addition of the base only slightly increased the pH of the solution from 4.74 to 4.75.
(c) Since HCl is a strong acid, it ionizes completely, forming 1.8×10–5 M H3O+ – notice that this dilute, unbuffered solution of HCl has the same hydronium ion concentration as the acetic acid-acetate buffer solution described in part (a). Therefore, we can deduce that the starting pH of the HCl solution is identical, 4.74.
First, we determine the number of moles of HCl present:
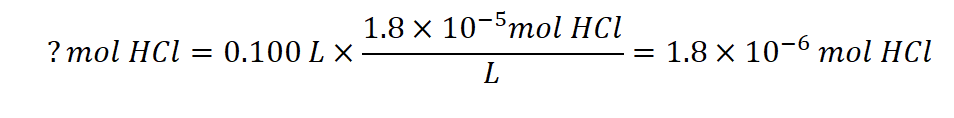
As shown in part (b), 1 mL of 0.10 M NaOH contains 1.0 × 10−4 mol of NaOH. When the NaOH and HCl solutions are mixed, the HCl is the limiting reagent in the reaction. All of the HCl reacts, and the amount of NaOH that remains is:
mol NaOH leftover= total mol- reacted mol
(1.0×10-4)-(1.8×10-6) = 9.8×10-5 M
The concentration of NaOH is:
9.8×10-5 M NaOH/0.101L= 9.7×10-4 M
The pOH of this solution is:
pOH = -log[OH–] = -log(9.7×10-4) = 3.01
The pH is:
pH = 14.00-pOH = 10.99
The pH changes from 4.74 to 10.99 in this unbuffered solution. This drastically differs from to the change of 4.74 to 4.75 that occurred when the same amount of NaOH was added to the buffered solution described in part (b).
Check Your Learning 6.2.1 – pH Changes in Buffered and Unbuffered Solutions
Show that adding 1.0 mL of 0.10 M HCl changes the pH of 100 mL of a 1.8 × 10−5 M HCl solution from 4.74 to 3.00.
Solution
Initial pH of 1.8 × 10−5 M HCl:
pH = −log[H3O+] = −log[1.8 × 10−5] = 4.74
Moles of H3O+ in 100 mL of 1.8 × 10−5 M HCl:
1.8 × 10−5 mol/L × 0.100 L = 1.8 × 10−6 mol
Moles of H3O+ added by addition of 1.0 mL of 0.10 M HCl:
0.10 mol/L × 0.0010 L = 1.0 × 10−4 mol
Final pH after addition of 1.0 mL of 0.10 M HCl:
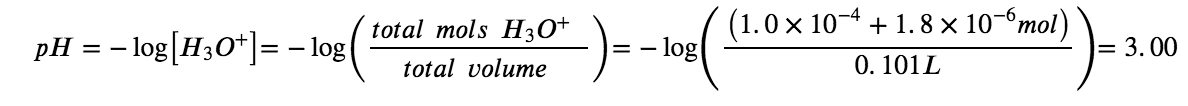
Selection of Suitable Buffer Mixtures
There are two useful rules of thumb for selecting buffer mixtures:
1. A good buffer mixture should have about equal concentrations of both of its components.
2. A buffer solution has generally lost its usefulness when the amount of one component of the buffer pair is less than about 10% of the other. Figure 6.2.2 shows the effect on the pH of an acetic acid-acetate ion buffer as the amount of strong base is added. Initially, the buffer contains equal amounts of acetic acid and acetate, and the initial pH is 4.74. A change of 1 pH unit occurs when the acetic acid concentration is reduced to 11% of the acetate ion concentration.
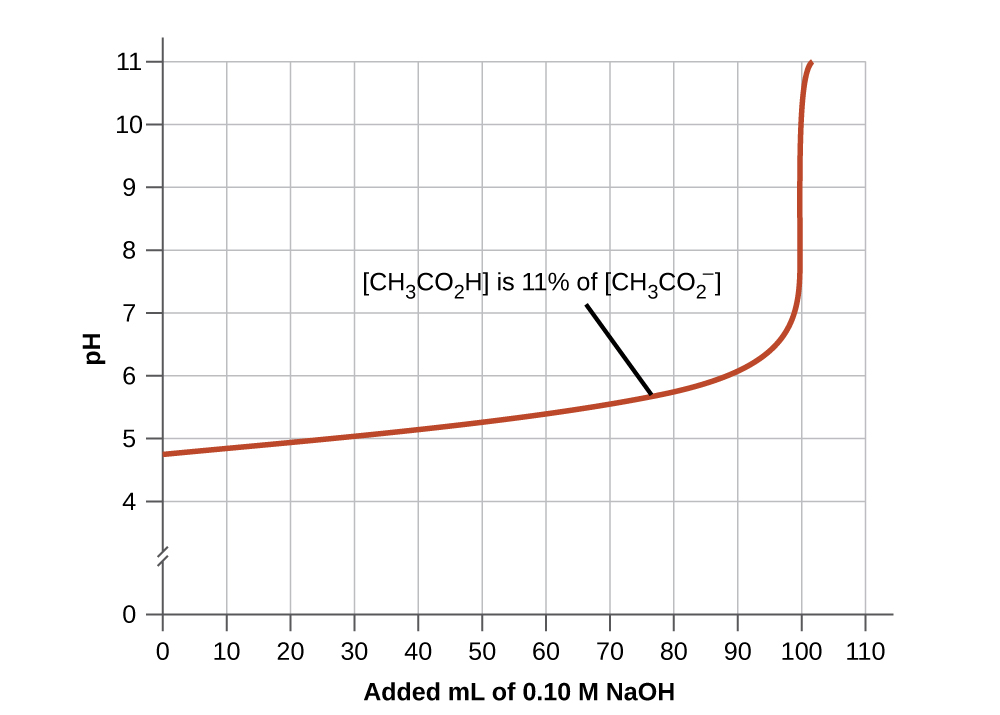
Figure 6.2.2. The graph, an illustration of buffering action, shows change of pH as an increasing amount of a 0.10 M NaOH solution is added to 100 mL of a buffer solution in which, initially, [CH3CO2H] = 0.10 M and [CH3CO2−] = 0.10 M.
3. Weak acids and their conjugate bases are better as buffers for pHs less than 7; weak bases and their conjugate acids are better as buffers for pHs greater than 7.
|
Buffering Action Curves Why do buffering action curves look the way they do? The key is to identify what happens at every part of the curve. Figure 6.2.2 shows an acetic acid-acetate ion buffer which begins at a pH of 4.74. As NaOH is slowly added the pH rises very little until about 90 mL NaOH is introduced. This is caused by the acetic acid reacting with the ionized OH– anions and the conjugate acid/base pair achieving equilibrium. This uses up the OH– in solution and keeps the pH fairly constant. After this, the graph shows us that the pH rises very quickly with a very small addition of NaOH. This is because the NaOH has used up all of the acetic acid available for neutralization of its OH– anion. Once there is no more neutralization possible, the excess OH– in solution quickly makes the solution basic with excess OH–. Although the graph cuts off, the end of the curve will plateau into a straight line. This occurs when sufficient NaOH has been added to bring the entire solution to a pH of approximately 13 (the pH of NaOH). |
The Henderson-Hasselbalch Equation
The ionization-constant expression for a solution of a weak acid can be written as:
Ka = ([H3O+][A–])/[HA]
Rearranging to solve for [H3O+], we get:
[H3O+] = Ka×([HA]/[A–])
Taking the negative logarithm of both sides of this equation, we arrive at:
-log[H3O+] = -log(Ka)-log([HA]/[A–])
which can be written as:
pH = pKa+log([A–]/[HA])
where pKa is the negative of the common logarithm of the ionization constant of the weak acid (pKa = −log(Ka)). This equation relates the pH, the ionization constant of a weak acid, and the concentrations of the weak acid and its conjugate base in a buffered solution. Scientists often use this expression, called the Henderson-Hasselbalch equation, to calculate the pH of buffer solutions. It is important to note that the “x is small” assumption must be valid to use this equation.
|
Lawrence Joseph Henderson and Karl Albert Hasselbalch Lawrence Joseph Henderson (1878–1942) was an American physician, biochemist and physiologist, to name only a few of his many pursuits. He obtained a medical degree from Harvard and then spent 2 years studying in Strasbourg, then a part of Germany, before returning to take a lecturer position at Harvard. He eventually became a professor at Harvard and worked there his entire life. He discovered that the acid-base balance in human blood is regulated by a buffer system formed by the dissolved carbon dioxide in blood. He wrote an equation in 1908 to describe the carbonic acid-bicarbonate buffer system in blood. Henderson was broadly knowledgeable; in addition to his important research on the physiology of blood, he also wrote on the adaptations of organisms and their fit with their environments, on sociology and on university education. He also founded the Fatigue Laboratory, at the Harvard Business School, which examined human physiology with specific focus on work in industry, exercise, and nutrition. In 1916, Karl Albert Hasselbalch (1874–1962), a Danish physician and chemist, shared authorship in a paper with Christian Bohr in 1904 that described the Bohr effect, which showed that the ability of hemoglobin in the blood to bind with oxygen was inversely related to the acidity of the blood and the concentration of carbon dioxide. The pH scale was introduced in 1909 by another Dane, Sørensen, and in 1912, Hasselbalch published measurements of the pH of blood. In 1916, Hasselbalch expressed Henderson’s equation in logarithmic terms, consistent with the logarithmic scale of pH, and thus the Henderson-Hasselbalch equation was born. |
Medicine: the Buffer System in Blood
Blood is an important example of a buffered solution, with the principal acid and ion responsible for the buffering action being carbonic acid, H2CO3, and the bicarbonate ion, HCO3−. When an excess of hydrogen ion enters the bloodstream, it is removed primarily by the reaction:
H3O+ (aq) + HCO3– (aq) → H2CO3 (aq) + H2O (l)
When an excess of the hydroxide ion is present, it is removed by the reaction:
OH– (aq) + H2CO3 (aq) → HCO3– (aq) + H2O (l)
The concentration of carbonic acid, H2CO3 is approximately 0.0012 M, and the concentration of the hydrogen carbonate ion, HCO3−, is around 0.024 M. Using the Henderson-Hasselbalch equation and the pKa of carbonic acid at body temperature, we can calculate the pH of blood:
pH = pKa+log([base]/[acid]) = 6.4+log(0.024/0.0012) = 7.7
The fact that the H2CO3 concentration is significantly lower than that of the HCO3− ion may seem unusual, but this imbalance is due to the fact that most of the by-products of our metabolism that enter our bloodstream are acidic. Therefore, there must be a larger proportion of base than acid, so that the capacity of the buffer will not be exceeded.
Lactic acid is produced in our muscles when we exercise. As the lactic acid enters the bloodstream, it is neutralized by the HCO3− ion, producing H2CO3. An enzyme then accelerates the breakdown of the excess carbonic acid to carbon dioxide and water, which can be eliminated by breathing. In fact, in addition to the regulating effects of the carbonate buffering system on the pH of blood, the body uses breathing to regulate blood pH. If the pH of the blood decreases too far, an increase in breathing removes CO2 from the blood through the lungs driving the equilibrium reaction such that [H3O+] is lowered. If the blood is too alkaline, a lower breath rate increases CO2 concentration in the blood, driving the equilibrium reaction the other way, increasing [H+] and restoring an appropriate pH.
Buffer Range and Buffer Capacity
Buffers are characterized by two parameters, buffer range and buffer capacity. Buffer range is the interval of pH values over which a buffer can reliably neutralize added external acids/bases. It is equal to pKa ± 1, and is thus a function of the identity of the acid in the conjugate acid/base pair used to construct the buffer. For example, the pKa of acetic acid is 4.74; therefore, buffers made by mixing acetic acid and acetate can have pH’s ranging from 3.74 up to 5.74. This is a consequence of the second requirement for buffers mentioned above: one component cannot be present in an amount that is less than 10% of the other, or:
1/10 < [base]/[acid] < 10/1
In other words, the ratio of base-to-acid in the buffer, must be greater than 0.1 but less than 10. As soon as this ratio becomes too lopsided (i.e. outside the above interval), then the buffer range has been exceeded, and we no longer expect the mixture to neutralize external acids/bases effectively.
Buffer solutions do not have an unlimited capacity to keep the pH relatively constant (Figure 6.2.3). If we add so much base to a buffer that the weak acid is exhausted, no more buffering action toward the base is possible. On the other hand, if we add an excess of acid, the weak base would be exhausted, and no more buffering action toward any additional acid would be possible. In fact, we do not even need to exhaust all of the acid or base in a buffer to overwhelm it; its buffering action will diminish rapidly as a given component nears depletion.

Figure 6.2.3. The indicator colour (methyl orange) shows that a small amount of acid added to a buffered solution of pH 8 (beaker on the left) has little effect on the buffered system (middle beaker). However, a large amount of acid exhausts the buffering capacity of the solution and the pH changes dramatically (beaker on the right). (credit: modification of work by Mark Ott)
The buffer capacity therefore is the amount of acid or base that can be added to a given volume of a buffer solution before the pH changes significantly, usually by one unit. Buffer capacity depends on the amounts of the weak acid and its conjugate base that are in a buffer mixture.
The more concentrated the buffer solution, the greater its buffer capacity. As illustrated in Figure 6.2.4, when NaOH is added to solutions that contain different concentrations of an acetic acid/sodium acetate buffer, the observed change in the pH of the buffer is inversely proportional to the concentration of the buffer. If the buffer capacity is 10 times larger, then the buffer solution can absorb 10 times more strong acid or base before undergoing a significant change in pH.
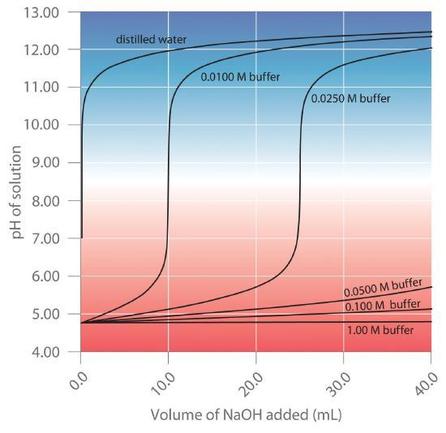
Figure 6.2.4. Effect of Buffer Concentration on the Capacity of a Buffer.
|
|
||||||||||||||||||||
|
Purpose In this experiment, you’ll need to refresh your memory of multiple concepts across various chapters (including this one): the core principles of equilibria and Le Châtelier’s Principle (Chapter 4), strong/weak acids and bases, dissociation constants, pH, and indicators (Chapter 5), and the purpose and function of buffers that we cover in this section (Chapter 6). This experiment is split into three main parts. First, you’ll be examining the effect of adding and consuming reactants on a [Cu(H2O)4]2+ / [Cu(NH3)4]2+ system at equilibrium in the context of Le Châtelier’s Principle. Following this, you’ll be establishing multiple equilibria using the Ag+ ion and noting observations when you add various reagents. Finally, you’ll prepare a buffer system that simulates the metabolic bicarbonate buffer system found in the blood of most animals, and determine what effects on the body are mimicked as you add acids and bases. Principles Equilibria pH Buffer systems Common-ion effect Temperature effect Safety Precautions Wear appropriate personal protective equipment (PPE) at all times in the laboratory setting – this includes your lab coat and safety goggles/glasses. Be sure to consult the MSDS for the following compounds for relevant health and safety, first aid, handling, and waste disposal information:
You’ll be working with acids and bases in the lab. Note that even when diluted, they are still corrosive and should be handled with caution. In case of contact with skin or clothing, notify your TA and make sure you rinse thoroughly with water for at least 15 minutes. If you happen to spill an acid or base, make sure you clean it up immediately since it can easily be mistaken for water (given that both are colourless). Handle silver nitrate with caution as it stains easily. Avoid deeply inhaling ammonia and sodium sulfide; both substances have very strong odours. You’ll be working with a lot of chemicals in this experiment, many of which are similar in appearance (e.g. many acids and bases appear colourless and non-viscous). Make sure you don’t confuse the reactants – check the label of each reagent you add when preparing the reaction mixture. Things to Consider As already emphasized in the experiment theory and instructions, all of the observations you’ll be taking are qualitative in nature, so make sure you record as many observations as possible! These include physical properties (e.g. phase of matter, colour, viscosity, solubility) and chemical properties (e.g. pH, reactivity with other substances). If needed, review your knowledge of acid/base equilibria, shifts in equilibrium systems and buffer systems. For practically all steps of this experiment, you add a reagent to a reaction mixture/system and you’ll need to explain the chemistry behind each step. To do so, you’ll need to 1) use the reaction equation provided in the instructions, and 2) integrate your knowledge of acid/base equilibria, equilibrium system and buffer systems. Reference Venkateswaran, R. General Chemistry – Laboratory Manual – CHM 1301/1311. |
||||||||||||||||||||
Mixture of appreciable amounts of a weak acid-base pair; the buffer resists dramatic changes in pH when small amounts of acid or base are added
Logarithmic version of the acid ionization constant expression, conveniently formatted for calculating the pH of buffer solutions
Amount of an acid or base that can be added to a volume of a buffer solution before its pH changes significantly (usually by one pH unit); in other words, the amount of acid or base a buffer can counteract

 CHM1311 Laboratory | Experiment #3: Equilibria
CHM1311 Laboratory | Experiment #3: Equilibria