9.2 – Ionic Bonding
As you have learned, ions are atoms or molecules bearing an electrical charge. A cation (a positive ion) forms when a neutral atom loses one or more electrons from its valence shell, and an anion (a negative ion) forms when a neutral atom gains one or more electrons in its valence shell.
Compounds composed of ions are called ionic compounds (or salts), and their constituent ions are held together by ionic bonds: electrostatic forces of attraction between oppositely charged cations and anions. The properties of ionic compounds shed some light on the nature of ionic bonds. Ionic solids exhibit a crystalline structure and tend to be rigid and brittle; they also tend to have high melting and boiling points, which suggests that ionic bonds are very strong. Ionic solids are also poor conductors of electricity for the same reason—the strength of ionic bonds prevents ions from moving freely in the solid state. Most ionic solids, however, dissolve readily in water. Once dissolved or melted, ionic compounds are excellent conductors of electricity and heat because the ions can move about freely.
Neutral atoms and their associated ions have very different physical and chemical properties. Sodium atoms form sodium metal, a soft, silvery-white metal that burns vigorously in air and reacts explosively with water. Chlorine atoms form chlorine gas, Cl2, a yellow-green gas that is extremely corrosive to most metals and very poisonous to animals and plants. The vigorous reaction between the elements sodium and chlorine forms the white, crystalline compound sodium chloride, common table salt, which contains sodium cations and chloride anions (Figure 9.2.1). The compound composed of these ions exhibits properties entirely different from the properties of the elements sodium and chlorine. Chlorine is poisonous, but sodium chloride is essential to life; sodium atoms react vigorously with water, but sodium chloride simply dissolves in water.
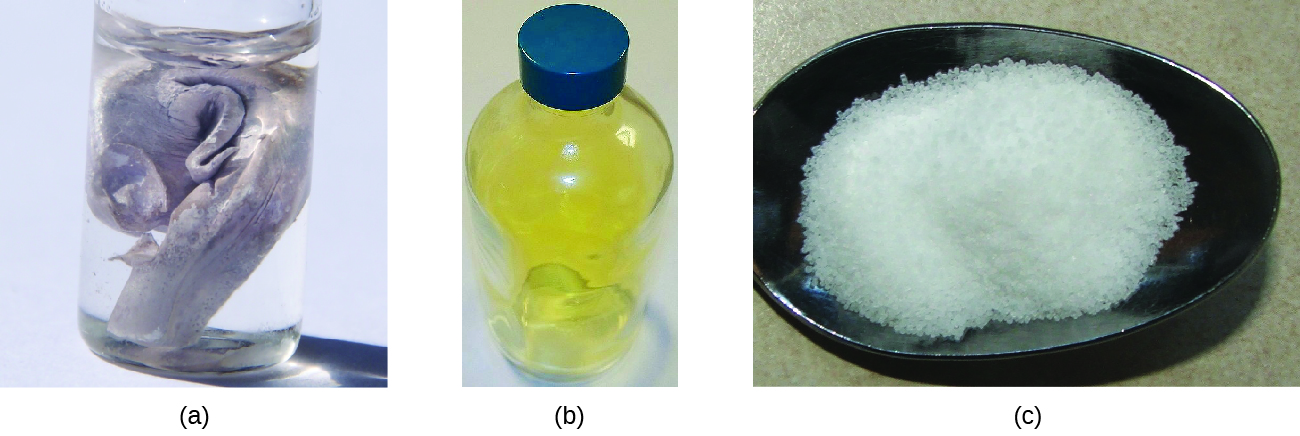
Figure 9.2.1. (a) Sodium is a soft metal that must be stored in mineral oil to prevent reaction with air or water. (b) Chlorine is a pale yellow-green gas. (c) When combined, they form white crystals of sodium chloride (table salt). (credit a: modification of work by “Jurii”/Wikimedia Commons)
It is important to note, however, that the formula for an ionic compound does not represent the physical arrangement of its ions. It is incorrect to refer to a sodium chloride (NaCl) “molecule” because there is not a single ionic bond, per se, between any specific pair of sodium and chloride ions. The attractive forces between ions are isotropic—the same in all directions—meaning that any particular ion is equally attracted to all of the nearby ions of opposite charge. This results in the ions arranging themselves into a tightly bound, three-dimensional lattice structure. Sodium chloride, for example, consists of a regular arrangement of equal numbers of Na+ cations and Cl– anions (Figure 9.2.2). Therefore, since it is impossible to define a “molecule” of an ionic compound, we use an empirical formula (e.g. NaCl) instead of a molecular formula to represent the unit cell, and thus the simplest whole number ratio of elements present in the crystal lattice.
Figure 9.2.2. The ionic compound NaCl forms when electrons from sodium atoms are transferred to chlorine atoms. The resulting Na+ and Cl− ions form a three-dimensional solid that is held together by attractive electrostatic interactions.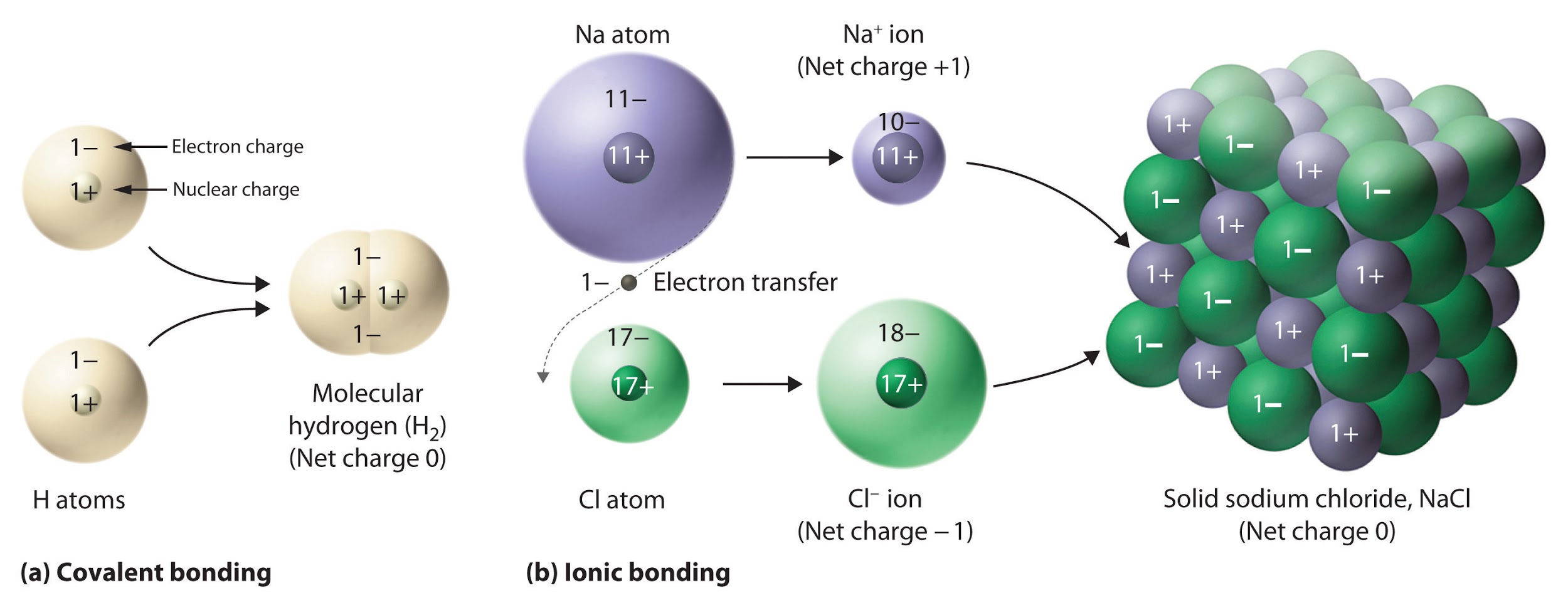
The strong electrostatic attraction between Na+ and Cl– ions holds them tightly together in solid NaCl. In the next section, we’ll examine this attractive force in detail.
Ionic Bond Strength and Lattice Energy
An ionic compound is stable because of the electrostatic attraction between its positive and negative ions. The lattice energy of a compound is a measure of the strength of this attraction. The lattice energy (ΔHlattice) of an ionic compound is defined as the energy required to separate one mole of the solid into its component gaseous ions. For the ionic solid MX, the lattice energy is the enthalpy change of the process:
MX (s) → Mn+ (g) + Xn- (g) ΔHlattice
Note that we are using the convention where the ionic solid is separated into ions, so our lattice energies will be endothermic (positive values). Some texts use the equivalent but opposite convention, defining lattice energy as the energy released when separate ions combine to form a lattice and giving negative (exothermic) values. Thus, if you are looking up lattice energies in another reference, be certain to check which definition is being used. In both cases, a larger magnitude for lattice energy indicates a more stable ionic compound. For sodium chloride, ΔHlattice = 769 kJ. Thus, it requires 769 kJ to separate one mole of solid NaCl into gaseous Na+ and Cl– ions. When one mole each of gaseous Na+ and Cl– ions form solid NaCl, 769 kJ of heat is released.
The lattice energy ΔHlattice of an ionic crystal can be expressed by the following equation (derived from Coulomb’s law, governing the forces between electric charges):
ΔHlattice = C(Z+)(Z–)R0
Equation 9.2.1 Lattice Energy of an Ionic Crystal
in which C is a constant that depends on the type of crystal structure; Z+ and Z– are the charges on the ions; and R0 is the interionic distance (the sum of the radii of the positive and negative ions). Thus, the lattice energy of an ionic crystal increases rapidly as the charges of the ions increase and the sizes of the ions decrease. When all other parameters are kept constant, doubling the charge of both the cation and anion quadruples the lattice energy. For example, the lattice energy of LiF (Z+ and Z– = 1) is 1023 kJ/mol, whereas that of MgO (Z+ and Z– = 2) is 3900 kJ/mol (Ro is nearly the same—about 200 pm for both compounds).
Different interatomic distances produce different lattice energies. For example, we can compare the lattice energy of MgF2 (2957 kJ/mol) to that of MgI2 (2327 kJ/mol) to observe the effect on lattice energy of the smaller ionic size of F– as compared to I–.
Example 9.2.1 – Lattice Energy Comparisons
The precious gem ruby is aluminum oxide, Al2O3, containing traces of Cr3+. The compound Al2Se3 is used in the fabrication of some semiconductor devices. Which has the larger lattice energy, Al2O3 or Al2Se3?
Solution
In these two ionic compounds, the charges Z+ and Z– are the same, so the difference in lattice energy will depend upon Ro. The O2– ion is smaller than the Se2– ion. Thus, Al2O3 would have a shorter interionic distance than Al2Se3, and Al2O3 would have the larger lattice energy.
Check Your Learning 9.2.1 – Lattice Energy Comparisons
Zinc oxide, ZnO, is a very effective sunscreen. How would the lattice energy of ZnO compare to that of NaCl? Refer to the figure below of ionic radii to assist you in answering this question.
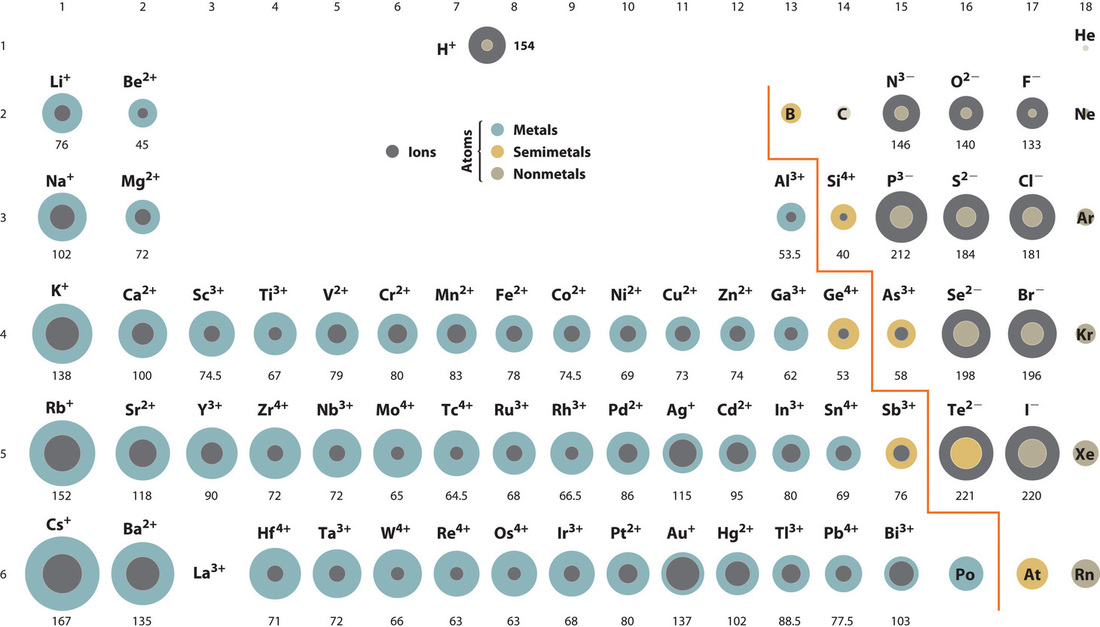
Figure 9.2.3. Periodic table with ionic radii of the most common ionic states of the elements, with the grey regions indicating the ionic radii and the colored regions the neutral radii.
Answer
ZnO would have the larger lattice energy because the Z values of both the cation and the anion in ZnO are greater, and the interionic distance of ZnO is smaller than that of NaCl.
★ Questions
1. Does a cation gain protons to form a positive charge or does it lose electrons?
2. Which of the following atoms would be expected to form negative ions in binary ionic compounds and which would be expected to form positive ions: P, I, Mg, Cl, In, Cs, O, Pb, Co?
3. Predict the charge on the monatomic ions formed from the following atoms in binary ionic compounds:
a) P
b) Mg
c) Al
d) O
e) Cl
f) Cs
4. Write the electron configuration for each of the following ions:
a) As3–
b) I–
c) Be2+
d) Cd2+
e) O2–
f) Ga3+
g) Li+
h) N3–
i) Sn2+
j) Co2+
k) Fe2+
l) As3+
5. Why is it incorrect to speak of a molecule of solid NaCl?
6. For which of the following substances is the least energy required to convert one mole of the solid into separate ions?
a) MgO
b) SrO
c) KF
d) CsF
e) MgF2
★★ Questions
7. The lattice energy of LiF is 1023 kJ/mol, and the Li–F distance is 201 pm. MgO crystallizes in the same structure as LiF but with a Mg–O distance of 205 pm. Which of the following values most closely approximates the lattice energy of MgO: 256 kJ/mol, 512 kJ/mol, 1023 kJ/mol, 2046 kJ/mol, or 4008 kJ/mol? Explain your choice.
8. Which compound in each of the following pairs has the larger lattice energy? Note: Ba2+ and K+ have similar radii; S2– and Cl– have similar radii. Explain your choices.
a) K2O or Na2O
b) K2S or BaS
c) KCl or BaS
d) BaS or BaCl2
Answers
1. The protons in the nucleus do not change during normal chemical reactions. Only the outer electrons move. Positive charges form when electrons are lost.
2. P, I, Cl, and O would form anions because they are nonmetals. Mg, In, Cs, Pb, and Co would form cations because they are metals.
3. (a) P3–; (b) Mg2+; (c) Al3+; (d) O2–; (e) Cl–; (f) Cs+
4. (a) [Ar]4s23d104p6; (b) [Kr]4d105s25p6 (c) 1s2 (d) [Kr]4d10; (e) [He]2s22p6; (f) [Ar]3d10; (g) 1s2 (h) [He]2s22p6 (i) [Kr]4d105s2 (j) [Ar]3d7 (k) [Ar]3d6, (l) [Ar]3d104s2
5. NaCl consists of discrete ions arranged in a crystal lattice, not covalently bonded molecules.
6. d)
7. 4008 kJ/mol; both ions in MgO have twice the charge of the ions in LiF; the bond length is very similar and both have the same structure; a quadrupling of the energy is expected based on the equation for lattice energy
8. (a) Na2O; Na+ has a smaller radius than K+; (b) BaS; Ba has a larger charge than K; (c) BaS; Ba and S have larger charges; (d) BaS; S has a larger charge
Energy required to separate one mole of an ionic solid into its component gaseous ions

