12. Mechanisms of Microbial Genetics
12.7 Gene Regulation
Learning Objectives
- Compare inducible operons and repressible operons
- Describe why regulation of operons is important
- Define quorum sensing and provide examples of the types of structures & behaviours regulated by quorum sensing
- Describe the paradigm lux system of quorum sensing
Each nucleated cell in a multicellular organism contains copies of the same DNA. Similarly, all cells in two pure bacterial cultures inoculated from the same starting colony contain the same DNA, with the exception of changes that arise from spontaneous mutations. If each cell in a multicellular organism has the same DNA, then how is it that cells in different parts of the organism’s body exhibit different characteristics? Similarly, how is it that the same bacterial cells within two pure cultures exposed to different environmental conditions can exhibit different phenotypes? In both cases, each genetically identical cell does not turn on, or express, the same set of genes. Only a subset of proteins in a cell at a given time is expressed.
Genomic DNA contains both structural genes, which encode products that serve as cellular structures or enzymes, and regulatory genes, which encode products that regulate gene expression. The expression of a gene is a highly regulated process. Whereas regulating gene expression in multicellular organisms allows for cellular differentiation, in single-celled organisms like prokaryotes, it primarily ensures that a cell’s resources are not wasted making proteins that the cell does not need at that time.
Elucidating the mechanisms controlling gene expression is important to the understanding of human health. Malfunctions in this process in humans lead to the development of cancer and other diseases. Understanding the interaction between the gene expression of a pathogen and that of its human host is important for the understanding of a particular infectious disease. Gene regulation involves a complex web of interactions within a given cell among signals from the cell’s environment, signalling molecules within the cell, and the cell’s DNA. These interactions lead to the expression of some genes and the suppression of others, depending on circumstances.
Prokaryotes and eukaryotes share some similarities in their mechanisms to regulate gene expression; however, gene expression in eukaryotes is more complicated because of the temporal and spatial separation between the processes of transcription and translation. Thus, although most regulation of gene expression occurs through transcriptional control in prokaryotes, regulation of gene expression in eukaryotes occurs at the transcriptional level and post-transcriptionally (after the primary transcript has been made).
Prokaryotic Gene Regulation
In bacteria and archaea, structural proteins with related functions are usually encoded together within the genome in a block called an operon and are transcribed together under the control of a single promoter, resulting in the formation of a polycistronic transcript (Figure 12.30). In this way, regulation of the transcription of all of the structural genes encoding the enzymes that catalyze the many steps in a single biochemical pathway can be controlled simultaneously, because they will either all be needed at the same time, or none will be needed. For example, in E. coli, all of the structural genes that encode enzymes needed to use lactose as an energy source lie next to each other in the lactose (or lac) operon under the control of a single promoter, the lac promoter. French scientists François Jacob (1920–2013) and Jacques Monod at the Pasteur Institute were the first to show the organization of bacterial genes into operons, through their studies on the lac operon of E. coli. For this work, they won the Nobel Prize in Physiology or Medicine in 1965. Although eukaryotic genes are not organized into operons, prokaryotic operons are excellent models for learning about gene regulation generally. There are some gene clusters in eukaryotes that function similar to operons. Many of the principles can be applied to eukaryotic systems and contribute to our understanding of changes in gene expression in eukaryotes that can result pathological changes such as cancer.
Each operon includes DNA sequences that influence its own transcription; these are located in a region called the regulatory region. The regulatory region includes the promoter and the region surrounding the promoter, to which transcription factors, proteins encoded by regulatory genes, can bind. Transcription factors influence the binding of RNA polymerase to the promoter and allow its progression to transcribe structural genes. A repressor is a transcription factor that suppresses transcription of a gene in response to an external stimulus by binding to a DNA sequence within the regulatory region called the operator, which is located between the RNA polymerase binding site of the promoter and the transcriptional start site of the first structural gene. Repressor binding physically blocks RNA polymerase from transcribing structural genes. Conversely, an activator is a transcription factor that increases the transcription of a gene in response to an external stimulus by facilitating RNA polymerase binding to the promoter. An inducer, a third type of regulatory molecule, is a small molecule that either activates or represses transcription by interacting with a repressor or an activator.
In prokaryotes, there are examples of operons whose gene products are required rather consistently and whose expression, therefore, is unregulated. Such operons are constitutively expressed, meaning they are transcribed and translated continuously to provide the cell with constant intermediate levels of the protein products. Such genes encode enzymes involved in housekeeping functions required for cellular maintenance, including DNA replication, repair, and expression, as well as enzymes involved in core metabolism. In contrast, there are other prokaryotic operons that are expressed only when needed and are regulated by repressors, activators, and inducers.
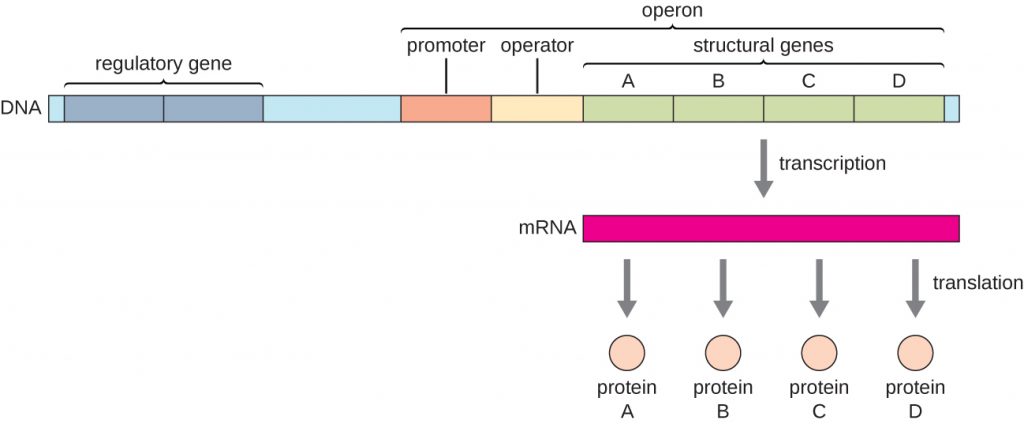
- What are the parts in the DNA sequence of an operon?
- What types of regulatory molecules are there?
Regulation by Repression
Prokaryotic operons are commonly controlled by the binding of repressors to operator regions, thereby preventing the transcription of the structural genes. Such operons are classified as either repressible operons or inducible operons. Repressible operons, like the tryptophan (trp) operon, typically contain genes encoding enzymes required for a biosynthetic pathway. As long as the product of the pathway, like tryptophan, continues to be required by the cell, a repressible operon will continue to be expressed. However, when the product of the biosynthetic pathway begins to accumulate in the cell, removing the need for the cell to continue to make more, the expression of the operon is repressed. Conversely, inducible operons, like the lac operon of E. coli, often contain genes encoding enzymes in a pathway involved in the metabolism of a specific substrate like lactose. These enzymes are only required when that substrate is available, thus expression of the operons is typically induced only in the presence of the substrate.
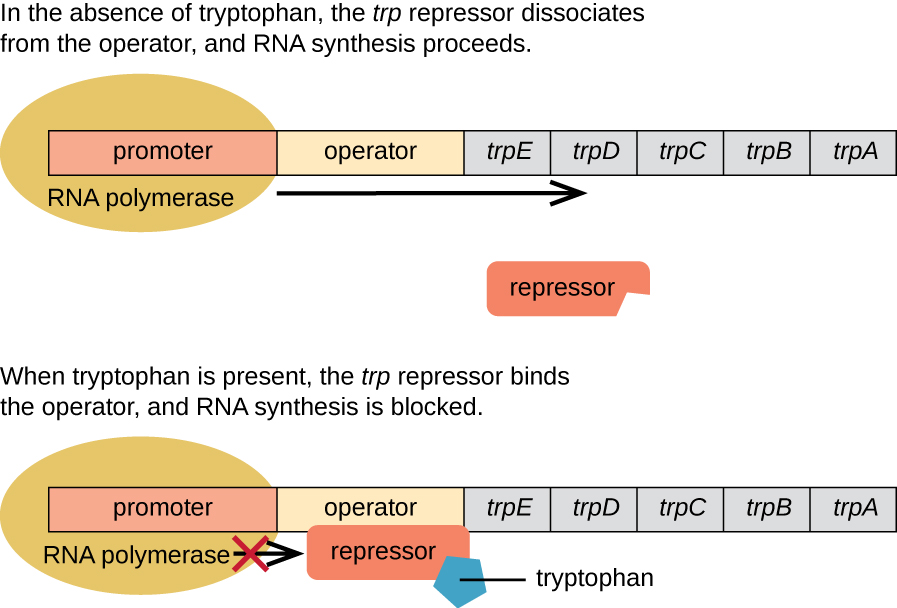
The trp Operon: A Repressible Operon
E. coli can synthesize tryptophan using enzymes that are encoded by five structural genes located next to each other in the trp operon (Figure 12.31). When environmental tryptophan is low, the operon is turned on. This means that transcription is initiated, the genes are expressed, and tryptophan is synthesized. However, if tryptophan is present in the environment, the trp operon is turned off. Transcription does not occur and tryptophan is not synthesized.
When tryptophan is not present in the cell, the repressor by itself does not bind to the operator; therefore, the operon is active and tryptophan is synthesized. However, when tryptophan accumulates in the cell, two tryptophan molecules bind to the trp repressor molecule, which changes its shape, allowing it to bind to the trp operator. This binding of the active form of the trp repressor to the operator blocks RNA polymerase from transcribing the structural genes, stopping expression of the operon. Thus, the actual product of the biosynthetic pathway controlled by the operon regulates the expression of the operon.
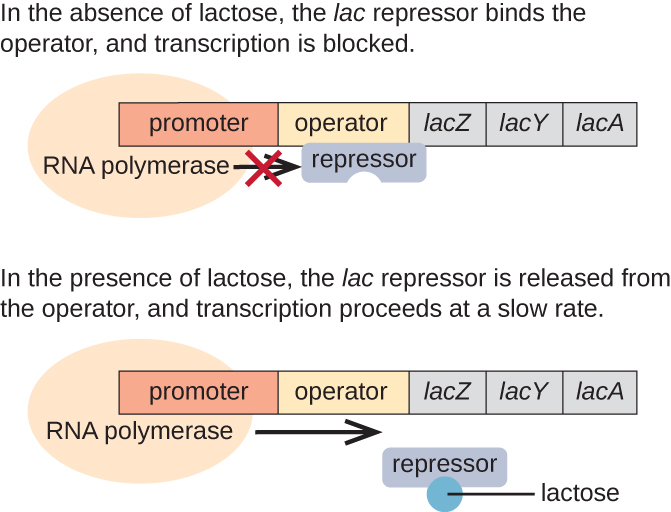
The lac Operon: An Inducible Operon
The lac operon is an example of an inducible operon that is also subject to activation in the absence of glucose (Figure 12.32). The lac operon encodes three structural genes necessary to acquire and process the disaccharide lactose from the environment, breaking it down into the simple sugars glucose and galactose. For the lac operon to be expressed, lactose must be present. This makes sense for the cell because it would be energetically wasteful to create the enzymes to process lactose if lactose was not available.
In the absence of lactose, the lac repressor is bound to the operator region of the lac operon, physically preventing RNA polymerase from transcribing the structural genes. However, when lactose is present, the lactose inside the cell is converted to allolactose. Allolactose serves as an inducer molecule, binding to the repressor and changing its shape so that it is no longer able to bind to the operator DNA. Removal of the repressor in the presence of lactose allows RNA polymerase to move through the operator region and begin transcription of the lac structural genes.
The lac Operon: Activation by Catabolite Activator Protein
Bacteria typically have the ability to use a variety of substrates as carbon sources. However, because glucose is usually preferable to other substrates, bacteria have mechanisms to ensure that alternative substrates are only used when glucose has been depleted. Additionally, bacteria have mechanisms to ensure that the genes encoding enzymes for using alternative substrates are expressed only when the alternative substrate is available. In the 1940s, Jacques Monod was the first to demonstrate the preference for certain substrates over others through his studies of E. coli’s growth when cultured in the presence of two different substrates simultaneously. Such studies generated diauxic growth curves, like the one shown in Figure 12.33. Although the preferred substrate glucose is used first, E. coli grows quickly and the enzymes for lactose metabolism are absent. However, once glucose levels are depleted, growth rates slow, inducing the expression of the enzymes needed for the metabolism of the second substrate, lactose. Notice how the growth rate in lactose is slower, as indicated by the lower steepness of the growth curve.
The ability to switch from glucose use to another substrate like lactose is a consequence of the activity of an enzyme called Enzyme IIA (EIIA). When glucose levels drop, cells produce less ATP from catabolism (see Catabolism of Carbohydrates), and EIIA becomes phosphorylated. Phosphorylated EIIA activates adenylyl cyclase, an enzyme that converts some of the remaining ATP to cyclic AMP (cAMP), a cyclic derivative of AMP and important signalling molecule involved in glucose and energy metabolism in E. coli. As a result, cAMP levels begin to rise in the cell (Figure 12.34).
The lac operon also plays a role in this switch from using glucose to using lactose. When glucose is scarce, the accumulating cAMP caused by increased adenylyl cyclase activity binds to catabolite activator protein (CAP), also known as cAMP receptor protein (CRP). The complex binds to the promoter region of the lac operon (Figure 12.35). In the regulatory regions of these operons, a CAP binding site is located upstream of the RNA polymerase binding site in the promoter. Binding of the CAP-cAMP complex to this site increases the binding ability of RNA polymerase to the promoter region to initiate the transcription of the structural genes. Thus, in the case of the lac operon, for transcription to occur, lactose must be present (removing the lac repressor protein) and glucose levels must be depleted (allowing binding of an activating protein). When glucose levels are high, there is catabolite repression of operons encoding enzymes for the metabolism of alternative substrates. Because of low cAMP levels under these conditions, there is an insufficient amount of the CAP-cAMP complex to activate transcription of these operons. Table 12.6 summarizes the regulation of the lac operon.
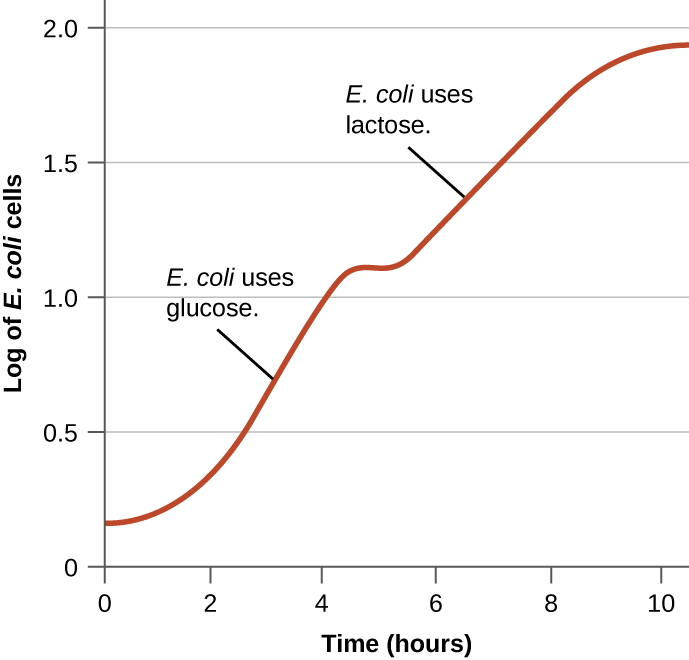
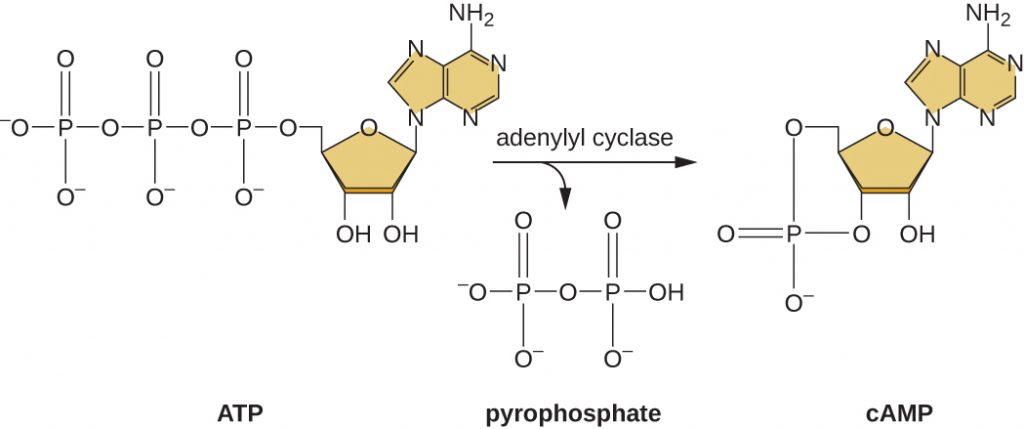
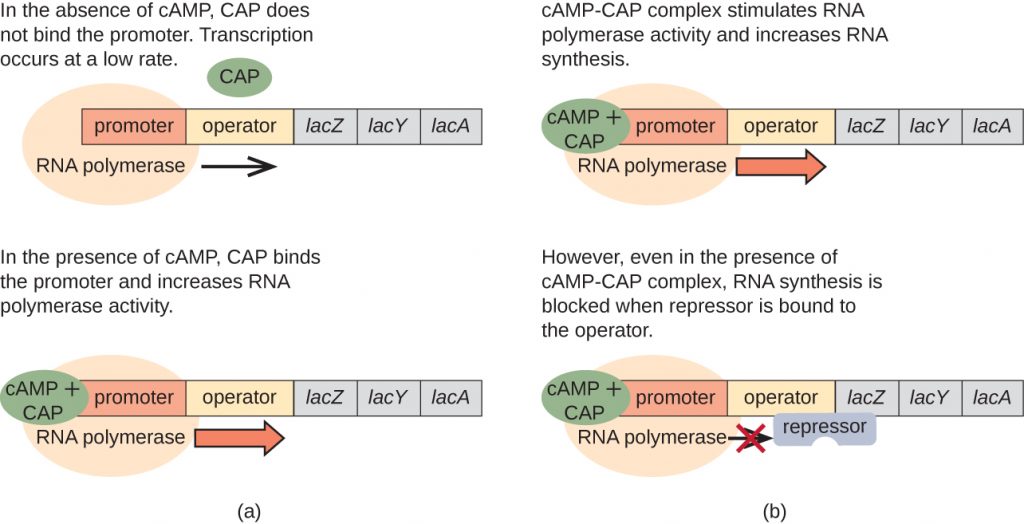
Table 12.6. Conditions Affecting Transcription of the lac Operon
| Conditions Affecting Transcription of the lac Operon | ||||
|---|---|---|---|---|
| Glucose | CAP binds | Lactose | Repressor binds | Transcription |
| + | – | – | + | No |
| + | – | + | – | Some |
| – | + | – | + | No |
| – | + | + | – | Yes |
- What affects the binding of the trp operon repressor to the operator?
- How and when is the behaviour of the lac repressor protein altered?
- In addition to being repressible, how else is the lac operon regulated?
Global Responses of Prokaryotes
In prokaryotes, there are also several higher levels of gene regulation that have the ability to control the transcription of many related operons simultaneously in response to an environmental signal. A group of operons all controlled simultaneously is called a regulon.
Alarmones
When sensing impending stress, prokaryotes alter the expression of a wide variety of operons to respond in coordination. They do this through the production of alarmones, which are small intracellular nucleotide derivatives. Alarmones change which genes are expressed and stimulate the expression of specific stress-response genes. The use of alarmones to alter gene expression in response to stress appears to be important in pathogenic bacteria. On encountering host defence mechanisms and other harsh conditions during infection, many operons encoding virulence genes are upregulated in response to alarmone signalling. Knowledge of these responses is key to being able to fully understand the infection process of many pathogens and to the development of therapies to counter this process.
Alternate σ Factors
Since the σ subunit of bacterial RNA polymerase confers specificity as to which promoters should be transcribed, altering the σ factor used is another way for bacteria to quickly and globally change what regulons are transcribed at a given time. The σ factor recognizes sequences within a bacterial promoter, so different σ factors will each recognize slightly different promoter sequences. In this way, when the cell senses specific environmental conditions, it may respond by changing which σ factor it expresses, degrading the old one and producing a new one to transcribe the operons encoding genes whose products will be useful under the new environmental condition. For example, in sporulating bacteria of the genera Bacillus and Clostridium (which include many pathogens), a group of σ factors controls the expression of the many genes needed for sporulation in response to sporulation-stimulating signals.
- What is the name given to a collection of operons that can be regulated as a group?
- What type of stimulus would trigger the transcription of a different σ factor?
Quorum Sensing
The first, and for the longest time, the only known example of bacterial density-dependent behaviour was the bioluminescence of Aliivibrio fischeri (previously Vibrio fischeri). This bacterium is a symbiont of the Hawaiian Bobtail Squid, growing in the host’s light organ until it reaches a critical population density, or “quorum”. This has become the paradigm system for quorum sensing, although there are other systems, including those that facilitate communication between different bacterial species. Regardless of which type of system, the basis for the ability of individual cells to detect the population density is based on secretion of a chemical or autoinducer (“AI”). With more cells, the concentration of autoinducer is also higher. The number of density-dependent behaviours are varied and wide-spread, but include bioluminescence, the production of antibiotics, biofilm formation, flagellation, conjugation, competence and expression of virulence factors.
The Paradigm Lux System
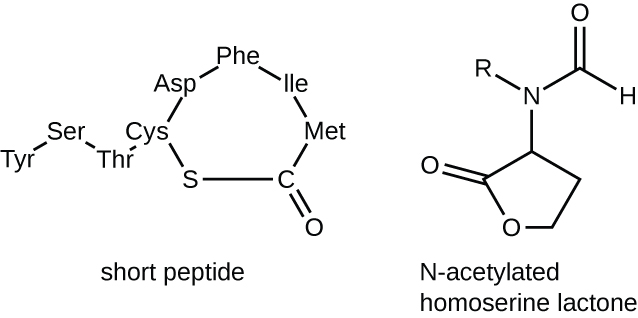
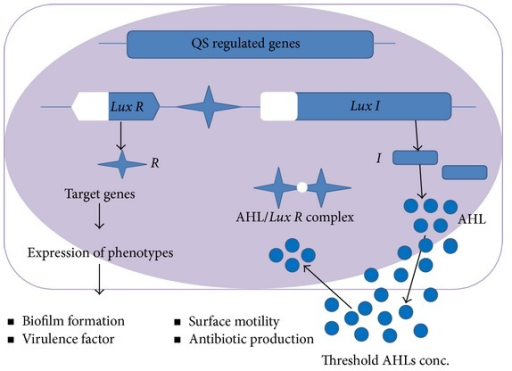
The Lux system controls bioluminescence in A. fischeri (“Lux” is a measure of illumination). It also is a common quorum sensing system in the Proteobacteria. The lux operon encodes an autoinducer synthase (LuxI) which synthesizes an acylhomoserine lactone autoinducer (AI) at a constant low rate (Figure 12.36). Gram positive bacteria use small signalling peptides in a different quorum sensing system.
AI is a small, diffusible molecule that diffuses out of, and into, cells, increasing in concentration as the population increases. Adjacent to the operon is a gene for the response regulator (LuxR), also expressed at a constant rate (Figure 12.37). Quorum is determined by intracellular concentrations and LuxR:AI binding kinetics. At a critical population density, intracellular AI concentrations lead to LuxR:AI binding. The LuxR:AI complex is a transcriptional activator, binding a recognition sequence, the lux box, in promoters for quorum-regulated genes and operons. This leads to much increased transcriptional levels of the lux operon and turns on (or occasionally off), other QS-regulated genes and operons. In the case of A. fischeri, transcriptional activation leads to increased luxI expression, and therefore AI production (hence the term “autoinducer”), but also induces luminescence through the activities of the remaining lux gene products.
Additional Methods of Regulation in Bacteria: Attenuation and Riboswitches
Although most gene expression is regulated at the level of transcription initiation in prokaryotes, there are also mechanisms to control both the completion of transcription as well as translation concurrently. Since their discovery, these mechanisms have been shown to control the completion of transcription and translation of many prokaryotic operons. Because these mechanisms link the regulation of transcription and translation directly, they are specific to prokaryotes, because these processes are physically separated in eukaryotes.
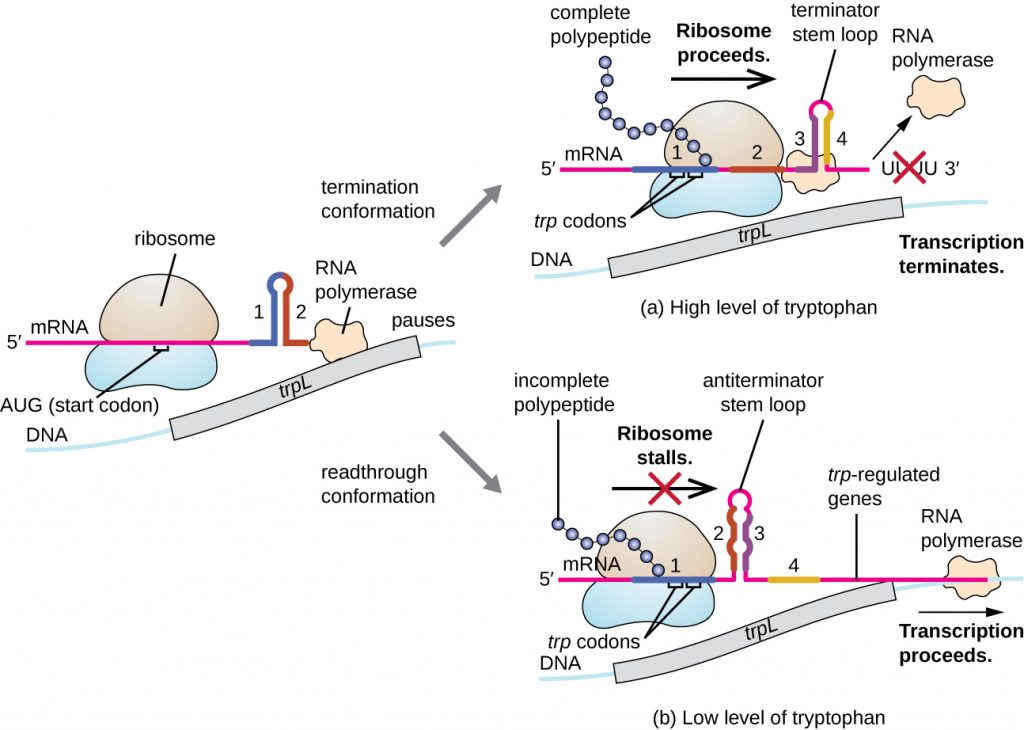
One such regulatory system is attenuation, whereby secondary stem-loop structures formed within the 5’ end of an mRNA being transcribed determine if transcription to complete the synthesis of this mRNA will occur and if this mRNA will be used for translation. Beyond the transcriptional repression mechanism already discussed, attenuation also controls expression of the trp operon in E. coli (Figure 12.39). The trp operon regulatory region contains a leader sequence called trpL between the operator and the first structural gene, which has four stretches of RNA that can base pair with each other in different combinations. When a terminator stem-loop forms, transcription terminates, releasing RNA polymerase from the mRNA. However, when an anti-terminator stem-loop forms, this prevents the formation of the terminator stem-loop, so RNA polymerase can transcribe the structural genes.
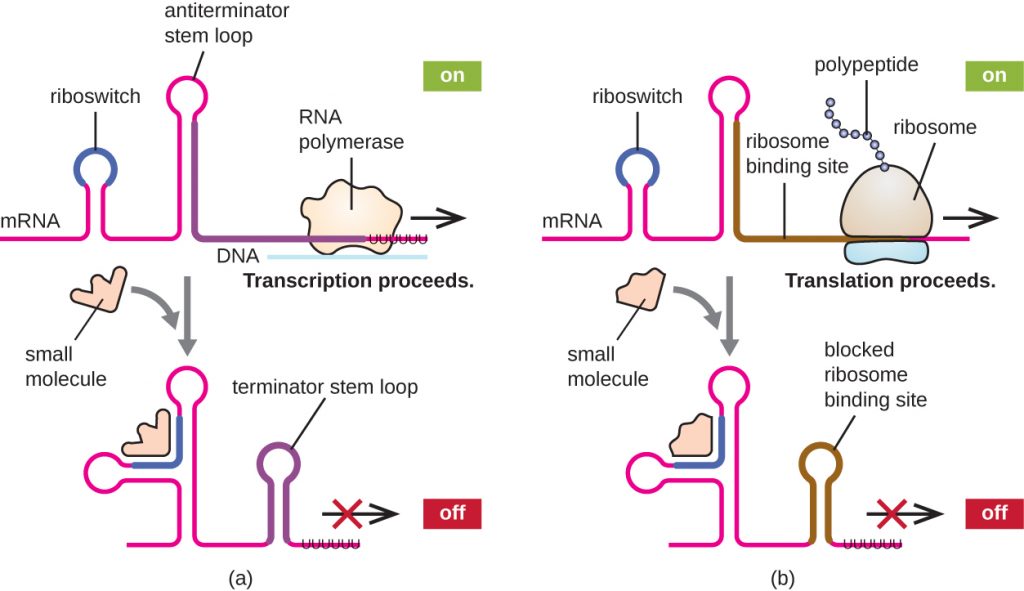
A related mechanism of concurrent regulation of transcription and translation in prokaryotes is the use of a riboswitch, a small region of noncoding RNA found within the 5’ end of some prokaryotic mRNA molecules (Figure 12.40). A riboswitch may bind to a small intracellular molecule to stabilize certain secondary structures of the mRNA molecule. The binding of the small molecule determines which stem-loop structure forms, thus influencing the completion of mRNA synthesis and protein synthesis.
Other Factors Affecting Gene Expression in Prokaryotes and Eukaryotes
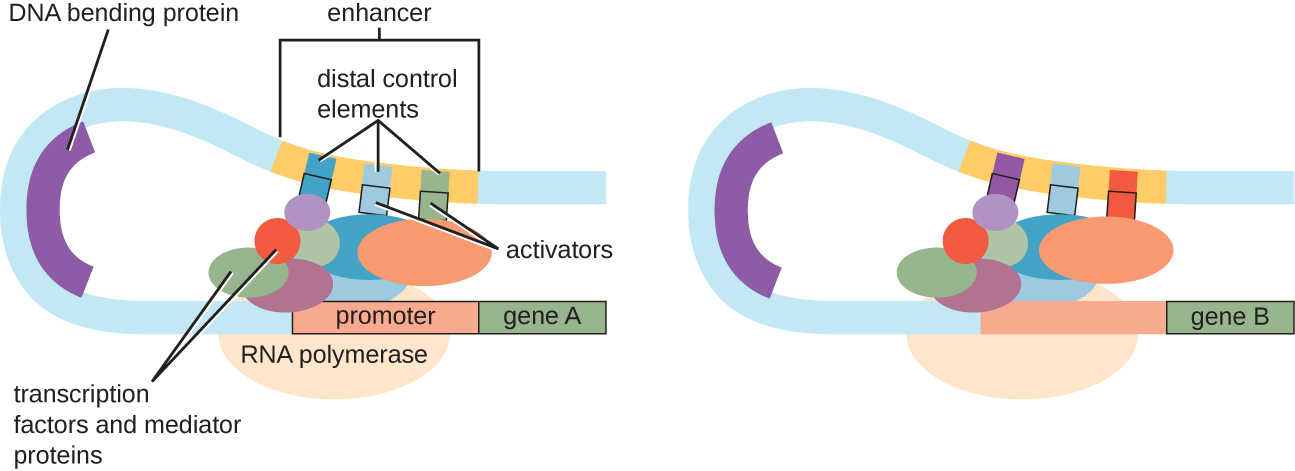
Although the focus on our discussion of transcriptional control used prokaryotic operons as examples, eukaryotic transcriptional control is similar in many ways. As in prokaryotes, eukaryotic transcription can be controlled through the binding of transcription factors including repressors and activators. Interestingly, eukaryotic transcription can be influenced by the binding of proteins to regions of DNA, called enhancers, rather far away from the gene, through DNA looping facilitated between the enhancer and the promoter (Figure 12.41). Overall, regulating transcription is a highly effective way to control gene expression in both prokaryotes and eukaryotes. However, the control of gene expression in eukaryotes in response to environmental and cellular stresses can be accomplished in additional ways without the binding of transcription factors to regulatory regions.
DNA-Level Control
In eukaryotes, the DNA molecules or associated histones can be chemically modified in such a way as to influence transcription; this is called epigenetic regulation. Methylation of certain cytosine nucleotides in DNA in response to environmental factors has been shown to influence use of such DNA for transcription, with DNA methylation commonly correlating to lowered levels of gene expression. Additionally, in response to environmental factors, histone proteins for packaging DNA can also be chemically modified in multiple ways, including acetylation and de-acetylation, influencing the packaging state of DNA and thus affecting the availability of loosely wound DNA for transcription. These chemical modifications can sometimes be maintained through multiple rounds of cell division, making at least some of these epigenetic changes heritable.
- What stops or allows transcription to proceed when attenuation is operating?
- What determines the state of a riboswitch?
- Describe the function of an enhancer.
- Describe two mechanisms of epigenetic regulation in eukaryotes.
CLINICAL FOCUS: Resolution
Although Mark survived his bout with necrotizing fasciitis, he would now have to undergo a skin-grafting surgery, followed by long-term physical therapy. Based on the amount of muscle mass he lost, it is unlikely that his leg will return to full strength, but his physical therapist is optimistic that he will regain some use of his leg.
Laboratory testing revealed the causative agent of Mark’s infection was a strain of group A streptococcus (Group A strep). As required by law, Mark’s case was reported to the state health department and ultimately to the Centers for Disease Control and Prevention (CDC). At the CDC, the strain of group A strep isolated from Mark was analyzed more thoroughly for methicillin resistance.
Methicillin resistance is genetically encoded and is becoming more common in group A strep through horizontal gene transfer. In necrotizing fasciitis, blood flow to the infected area is typically limited because of the action of various genetically encoded bacterial toxins. This is why there is typically little to no bleeding as a result of the incision test. Unfortunately, these bacterial toxins limit the effectiveness of intravenous antibiotics in clearing infection from the skin and underlying tissue, meaning that antibiotic resistance alone does not explain the ineffectiveness of Mark’s treatment. Nevertheless, intravenous antibiotic therapy was warranted to help minimize the possible outcome of sepsis, which is a common outcome of necrotizing fasciitis. Through genomic analysis by the CDC of the strain isolated from Mark, several of the important virulence genes were shown to be encoded on prophages, indicating that transduction is important in the horizontal gene transfer of these genes from one bacterial cell to another.
Go back to the previous Clinical Focus box.
Key Takeaways
- Gene expression is a tightly regulated process.
- Gene expression in prokaryotes is largely regulated at the point of transcription. Gene expression in eukaryotes is additionally regulated post-transcriptionally.
- Prokaryotic structural genes of related function are often organized into operons, all controlled by transcription from a single promoter. The regulatory region of an operon includes the promoter itself and the region surrounding the promoter to which transcription factors can bind to influence transcription.
- Although some operons are constitutively expressed, most are subject to regulation through the use of transcription factors (repressors and activators). A repressor binds to an operator, a DNA sequence within the regulatory region between the RNA polymerase binding site in the promoter and first structural gene, thereby physically blocking transcription of these operons. An activator binds within the regulatory region of an operon, helping RNA polymerase bind to the promoter, thereby enhancing the transcription of this operon. An inducer influences transcription through interacting with a repressor or activator.
- The trp operon is a classic example of a repressible operon. When tryptophan accumulates, tryptophan binds to a repressor, which then binds to the operator, preventing further transcription.
- The lac operon is a classic example an inducible operon. When lactose is present in the cell, it is converted to allolactose. Allolactose acts as an inducer, binding to the repressor and preventing the repressor from binding to the operator. This allows transcription of the structural genes.
- The lac operon is also subject to activation. When glucose levels are depleted, some cellular ATP is converted into cAMP, which binds to the catabolite activator protein (CAP). The cAMP-CAP complex activates transcription of the lac operon. When glucose levels are high, its presence prevents transcription of the lac operon and other operons by catabolite repression.
- Small intracellular molecules called alarmones are made in response to various environmental stresses, allowing bacteria to control the transcription of a group of operons, called a regulon.
- Bacteria have the ability to change which σ factor of RNA polymerase they use in response to environmental conditions to quickly and globally change which regulons are transcribed.
- Prokaryotes have regulatory mechanisms, including attenuation and the use of riboswitches, to simultaneously control the completion of transcription and translation from that transcript. These mechanisms work through the formation of stem loops in the 5’ end of an mRNA molecule currently being synthesized.
- There are additional points of regulation of gene expression in prokaryotes and eukaryotes. In eukaryotes, epigenetic regulation by chemical modification of DNA or histones, and regulation of RNA processing are two methods.
Multiple Choice
Fill in the Blank
Short Answer
- What are two ways that bacteria can influence the transcription of multiple different operons simultaneously in response to a particular environmental condition?
Critical Thinking
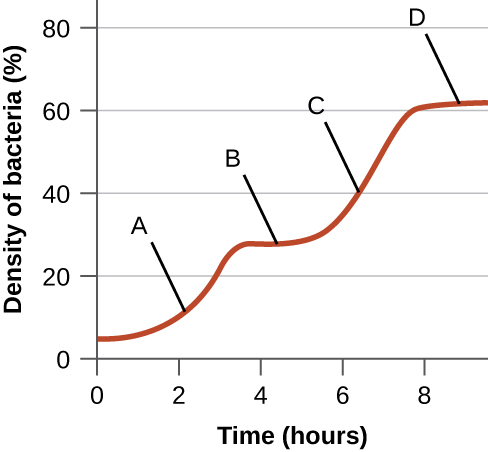
- The following figure is from Monod’s original work on diauxic growth showing the growth of E. coli in the simultaneous presence of xylose and glucose as the only carbon sources. Explain what is happening at points A–D with respect to the carbon source being used for growth, and explain whether the xylose-use operon is being expressed (and why). Note that expression of the enzymes required for xylose use is regulated in a manner similar to the expression of the enzymes required for lactose use.
Media Attributions
- OSC_Microbio_11_07_Operon
- OSC_Microbio_11_07_trp
- microbiology sign © Nick Youngson
- OSC_Microbio_11_07_lacrep
- OSC_Microbio_11_07_Diaux
- OSC_Microbio_11_07_ATPcAMP
- OSC_Microbio_11_07_CAP
- OSC_Microbio_09_05_autoinduc
- Quorum sensing paradigm
- OSC_Microbio_11_07_Atten
- OSC_Microbio_11_07_Riboswitch
- OSC_Microbio_11_07_Enhancer
- OSC_Microbio_11_07_Diaux_img


