27. Nervous System Infections
27.4 Fungal and Parasitic Diseases of the Nervous System
Learning Objectives
- Identify the most common fungi that can cause infections of the nervous system
- Compare the major characteristics of specific fungal diseases affecting the nervous system
Fungal infections of the nervous system, called neuromycoses, are rare in healthy individuals. However, neuromycoses can be devastating in immunocompromised or elderly patients. Several eukaryotic parasites are also capable of infecting the nervous system of human hosts. Although relatively uncommon, these infections can also be life-threatening in immunocompromised individuals. In this section, we will first discuss neuromycoses, followed by parasitic infections of the nervous system.
Cryptococcocal Meningitis
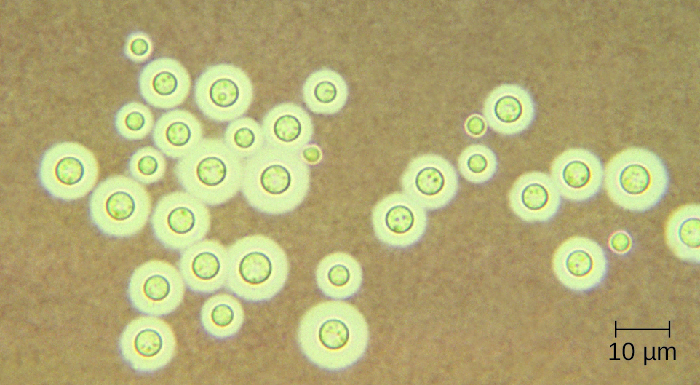
Cryptococcus neoformans is a fungal pathogen that can cause meningitis. This yeast is commonly found in soils and is particularly associated with pigeon droppings. It has a thick capsule that serves as an important virulence factor, inhibiting clearance by phagocytosis. Most C. neoformans cases result in subclinical respiratory infections that, in healthy individuals, generally resolve spontaneously with no long-term consequences (see Respiratory Mycoses). In immunocompromised patients or those with other underlying illnesses, the infection can progress to cause meningitis and granuloma formation in brain tissues. Cryptococcus antigens can also serve to inhibit cell-mediated immunity and delayed-type hypersensitivity.
Cryptococcus can be easily cultured in the laboratory and identified based on its extensive capsule (Figure 27.18). C. neoformans is frequently cultured from urine samples of patients with disseminated infections.
Prolonged treatment with antifungal drugs is required to treat cryptococcal infections. Combined therapy is required with amphotericin B plus flucytosine for at least 10 weeks. Many antifungal drugs have difficulty crossing the blood-brain barrier and have strong side effects that necessitate low doses; these factors contribute to the lengthy time of treatment. Patients with AIDS are particularly susceptible to Cryptococcus infections because of their compromised immune state. AIDS patients with cryptococcosis can also be treated with antifungal drugs, but they often have relapses; lifelong doses of fluconazole may be necessary to prevent reinfection.
- Why are neuromycoses infections rare in the general population?
- How is a cryptococcal infection acquired?
DISEASE PROFILE: Neuromycoses
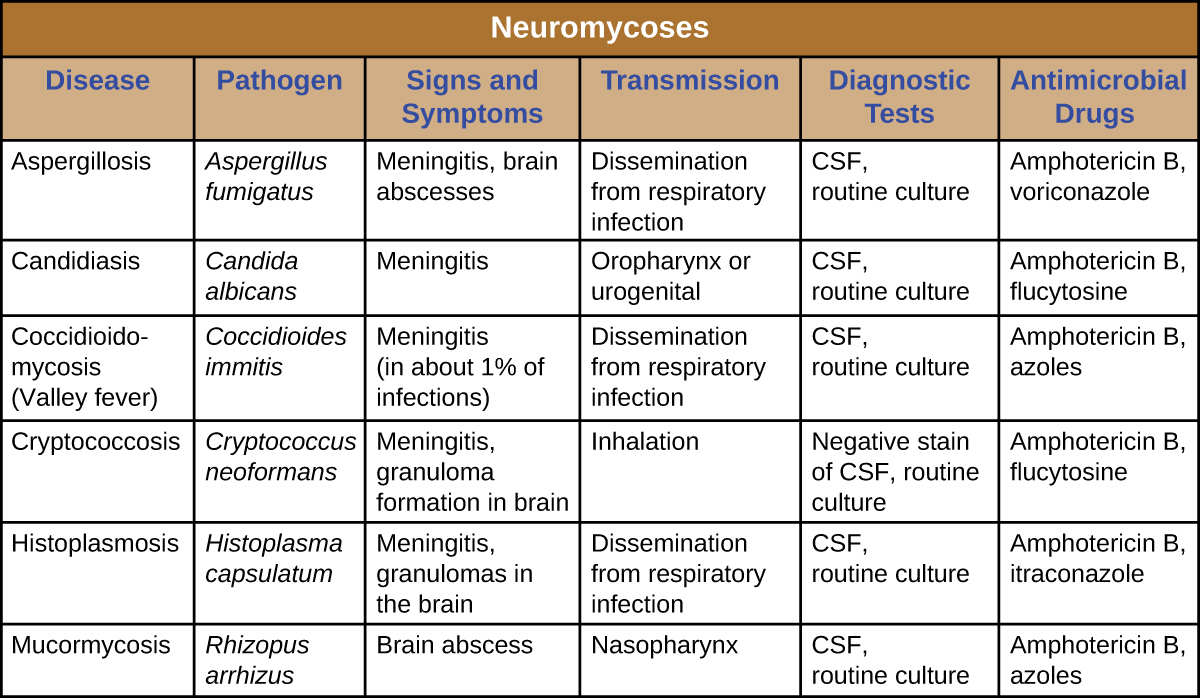
Neuromycoses typically occur only in immunocompromised individuals and usually only invade the nervous system after first infecting a different body system. As such, many diseases that sometimes affect the nervous system have already been discussed in previous chapters. Table 27.3 presents some of the most common fungal infections associated with neurological disease. This table includes only the neurological aspects associated with these diseases; it does not include characteristics associated with other body systems.
Table 27.3
CLINICAL FOCUS: Resolution
David’s new prescription for two antifungal drugs, amphotericin B and flucytosine, proved effective, and his condition began to improve. Culture results from David’s sputum, skin, and CSF samples confirmed a fungal infection. All were positive for C. neoformans. Serological tests of his tissues were also positive for the C. neoformans capsular polysaccharide antigen.
Since C. neoformans is known to occur in bird droppings, it is likely that David had been exposed to the fungus while working on the barn. Despite this exposure, David’s doctor explained to him that immunocompetent people rarely contract cryptococcal meningitis and that his immune system had likely been compromised by the anti-inflammatory medication he was taking to treat his Crohn’s disease. However, to rule out other possible causes of immunodeficiency, David’s doctor recommended that he be tested for HIV.
After David tested negative for HIV, his doctor took him off the corticosteroid he was using to manage his Crohn’s disease, replacing it with a different class of drug. After several weeks of antifungal treatments, David managed a full recovery.
Jump to the previous Clinical Focus box.
Amoebic Meningitis
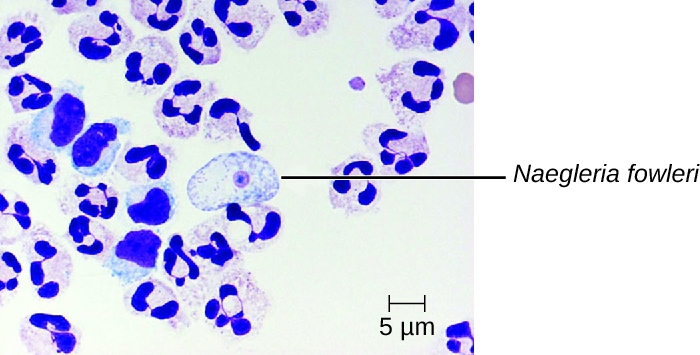
Primary amoebic meningoencephalitis (PAM) is caused by Naegleria fowleri. This amoeboflagellate is commonly found free-living in soils and water. It can exist in one of three forms—the infective amoebic trophozoite form, a motile flagellate form, and a resting cyst form. PAM is a rare disease that has been associated with young and otherwise healthy individuals. Individuals are typically infected by the amoeba while swimming in warm bodies of freshwater such as rivers, lakes, and hot springs. The pathogenic trophozoite infects the brain by initially entering through nasal passages to the sinuses; it then moves down olfactory nerve fibres to penetrate the submucosal nervous plexus, invades the cribriform plate, and reaches the subarachnoid space. The subarachnoid space is highly vascularized and is a route of dissemination of trophozoites to other areas of the CNS, including the brain (Figure 27.19). Inflammation and destruction of grey matter leads to severe headaches and fever. Within days, confusion and convulsions occur and quickly progress to seizures, coma, and death. The progression can be very rapid, and the disease is often not diagnosed until autopsy.
N. fowleri infections can be confirmed by direct observation of CSF; the amoebae can often be seen moving while viewing a fresh CSF wet mount through a microscope. Flagellated forms can occasionally also be found in CSF. The amoebae can be stained with several stains for identification, including Giemsa-Wright or a modified trichrome stain. Detection of antigens with indirect immunofluorescence, or genetic analysis with PCR, can be used to confirm an initial diagnosis. N. fowleri infections are nearly always fatal; only 3 of 138 patients with PAM in the United States have survived.[1] A new experimental drug called miltefosine shows some promise for treating these infections. This drug is a phosphotidylcholine derivative that is thought to inhibit membrane function in N. fowleri, triggering apoptosis and disturbance of lipid-dependent cell signalling pathways.[2] When administered early in infection and coupled with therapeutic hypothermia (lowering the body’s core temperature to reduce the cerebral oedema associated with infection), this drug has been successfully used to treat primary amoebic encephalitis.
Granulomatous Amoebic Encephalitis
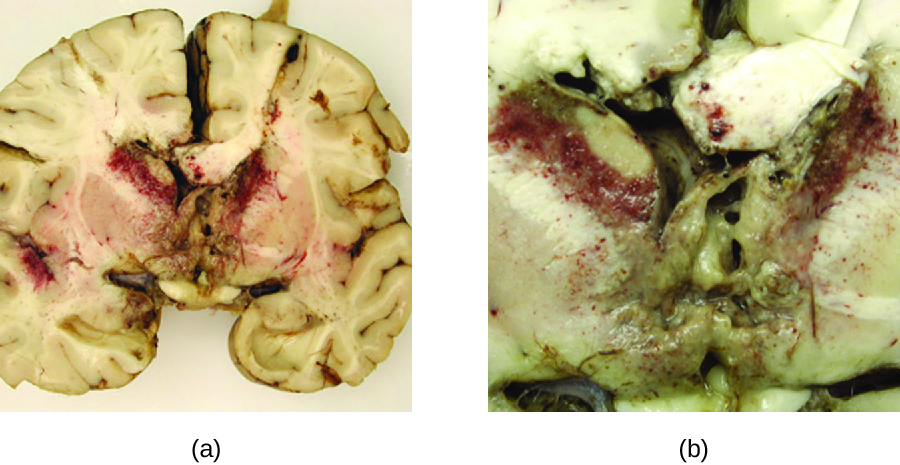
Acanthamoeba and Balamuthia species are free-living amoebae found in many bodies of fresh water. Human infections by these amoebae are rare. However, they can cause amoebic keratitis in contact lens wearers (see Protozoan Infections of the Eyes), disseminated infections in immunocompromised patients, and granulomatous amoebic encephalitis (GAE) in severe cases. Compared to PAM, GAE tend to be subacute infections. The microbe is thought to enter through either the nasal sinuses or breaks in the skin. It is disseminated hematogenously and can invade the CNS. There, the infections lead to inflammation, formation of lesions, and development of typical neurological symptoms of encephalitis (Figure 27.20). GAE is nearly always fatal.
GAE is often not diagnosed until late in the infection. Lesions caused by the infection can be detected using CT or MRI. The live amoebae can be directly detected in CSF or tissue biopsies. Serological tests are available but generally are not necessary to make a correct diagnosis, since the presence of the organism in CSF is definitive. Some antifungal drugs, like fluconazole, have been used to treat acanthamoebal infections. In addition, a combination of miltefosine and voriconazole (an inhibitor of ergosterol biosynthesis) has recently been used to successfully treat GAE. Even with treatment, however, the mortality rate for patients with these infections is high.
- How is granulomatous amoebic encephalitis diagnosed?
Human African Trypanosomiasis
Human African trypanosomiasis (also known as African sleeping sickness) is a serious disease endemic to two distinct regions in sub-Saharan Africa. It is caused by the insect-borne hemoflagellate Trypanosoma brucei. The subspecies Trypanosoma brucei rhodesiense causes East African trypanosomiasis (EAT), and another subspecies, Trypanosoma brucei gambiense causes West African trypanosomiasis (WAT). A few hundred cases of EAT are currently reported each year.[3] WAT is more commonly reported and tends to be a more chronic disease. Around 7000 to 10,000 new cases of WAT are identified each year.[4]
T. brucei is primarily transmitted to humans by the bite of the tsetse fly (Glossina spp.). Soon after the bite of a tsetse fly, a chancre forms at the site of infection. The flagellates then spread, moving into the circulatory system (Figure 27.21). These systemic infections result in an undulating fever, during which symptoms persist for two or three days with remissions of about a week between bouts. As the disease enters its final phase, the pathogens move from the lymphatics into the CNS. Neurological symptoms include daytime sleepiness, insomnia, and mental deterioration. In EAT, the disease runs its course over a span of weeks to months. In contrast, WAT often occurs over a span of months to years.
Although a strong immune response is mounted against the trypanosome, it is not sufficient to eliminate the pathogen. Through antigenic variation, Trypanosoma can change their surface proteins into over 100 serological types. This variation leads to the undulating form of the initial disease. The initial septicaemia caused by the infection leads to high fevers. As the immune system responds to the infection, the number of organisms decrease, and the clinical symptoms abate. However, a subpopulation of the pathogen then alters its surface coat antigens by antigenic variation and evades the immune response. These flagellates rapidly proliferate and cause another bout of disease. If untreated, these infections are usually fatal.
Clinical symptoms can be used to recognize the early signs of African trypanosomiasis. These include the formation of a chancre at the site of infection and Winterbottom’s sign. Winterbottom’s sign refers to the enlargement of lymph nodes on the back of the neck—often indicative of cerebral infections. Trypanosoma can be directly observed in stained samples including blood, lymph, CSF, and skin biopsies of chancres from patients. Antibodies against the parasite are found in most patients with acute or chronic disease. Serologic testing is generally not used for diagnosis, however, since the microscopic detection of the parasite is sufficient. Early diagnosis is important for treatment. Before the nervous system is involved, drugs like pentamidine (an inhibitor of nuclear metabolism) and suramin (mechanism unclear) can be used. These drugs have fewer side effects than the drugs needed to treat the second stage of the disease. Once the sleeping sickness phase has begun, harsher drugs including melarsoprol (an arsenic derivative) and eflornithine can be effective. Following successful treatment, patients still need to have follow-up examinations of their CSF for two years to detect possible relapses of the disease. The most effective means of preventing these diseases is to control the insect vector populations.
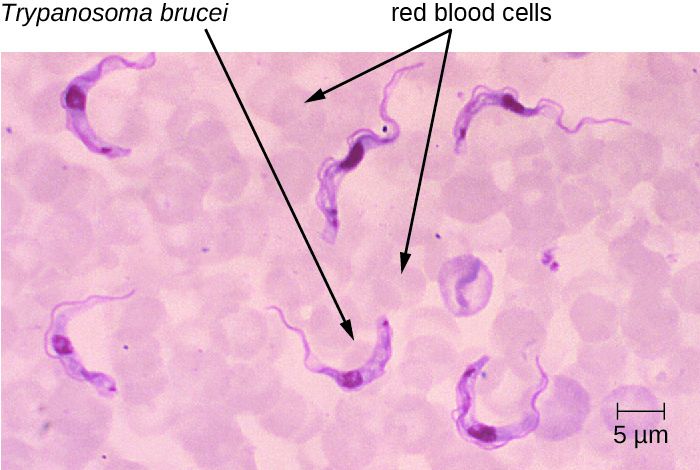
- What is the symptom of a systemic Trypanosoma infection?
- What are the symptoms of a neurological Trypanosoma infection?
- Why are trypanosome infections so difficult to eradicate?
Neurotoxoplasmosis
Toxoplasma gondii is an ubiquitous intracellular parasite that can cause neonatal infections. Cats are the definitive host, and humans can become infected after eating infected meat or, more commonly, by ingesting oocysts shed in the faeces of cats (see Parasitic Infections of the Circulatory and Lymphatic Systems). T. gondii enters the circulatory system by passing between the endothelial cells of blood vessels.[5] Most cases of toxoplasmosis are asymptomatic. However, in immunocompromised patients, neurotoxoplasmosis caused by T. gondii infections are one of the most common causes of brain abscesses.[6] The organism is able to cross the blood-brain barrier by infecting the endothelial cells of capillaries in the brain. The parasite reproduces within these cells, a step that appears to be necessary for entry to the brain, and then causes the endothelial cell to lyse, releasing the progeny into brain tissues. This mechanism is quite different than the method it uses to enter the bloodstream in the first place.[7]
The brain lesions associated with neurotoxoplasmosis can be detected radiographically using MRI or CAT scans (Figure 27.22). Diagnosis can be confirmed by direct observation of the organism in CSF. RT-PCR assays can also be used to detect T. gondii through genetic markers.
Treatment of neurotoxoplasmosis caused by T. gondii infections requires six weeks of multi-drug therapy with pyrimethamine, sulfadiazine, and folinic acid. Long-term maintenance doses are often required to prevent recurrence.
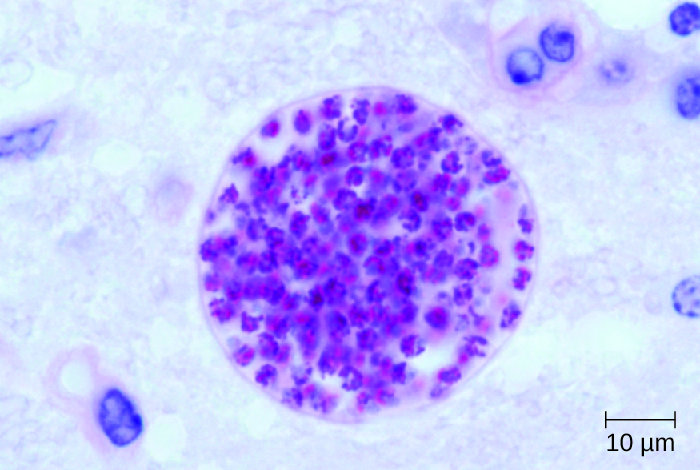
- Under what conditions is Toxoplasma infection serious?
- How does Toxoplasma circumvent the blood-brain barrier?
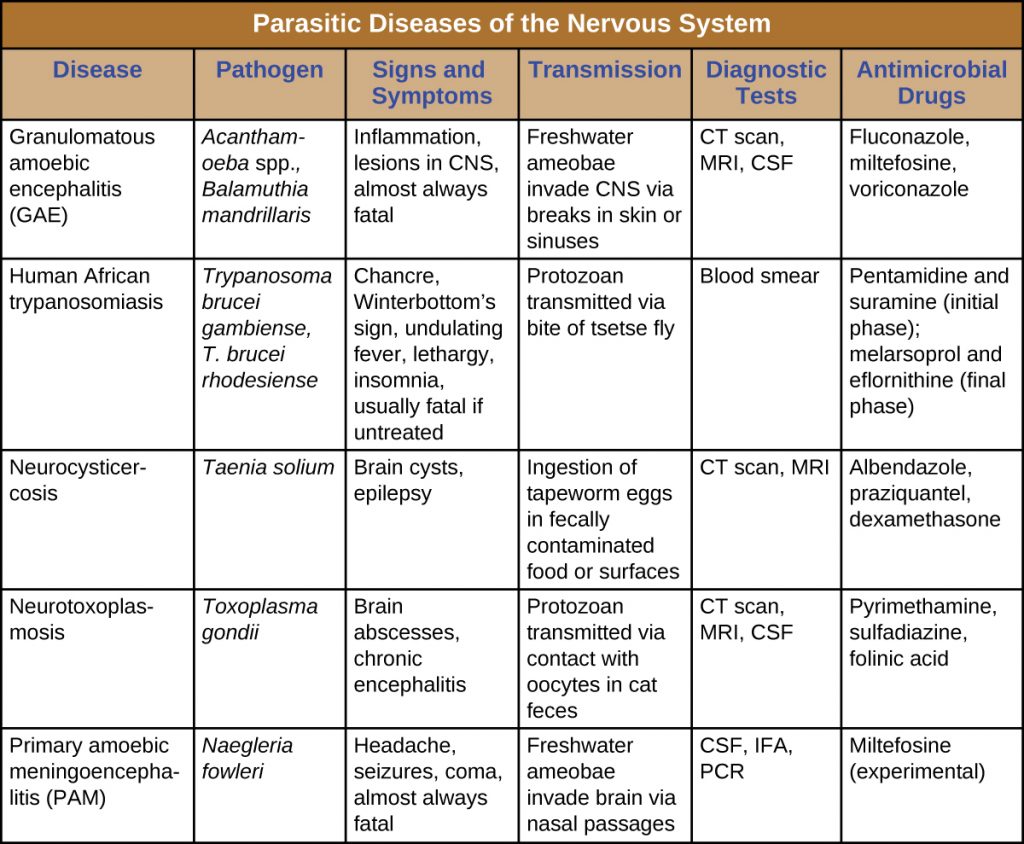
DISEASE PROFILE: Parasitic Diseases of the Nervous System
Parasites that successfully invade the nervous system can cause a wide range of neurological signs and symptoms. Often, they inflict lesions that can be visualized through radiologic imaging. A number of these infections are fatal, but some can be treated (with varying levels of success) by antimicrobial drugs (Table 27.3).
Table 27.3
Key Takeaways
- Neuromycoses are uncommon in immunocompetent people, but immunocompromised individuals with fungal infections have high mortality rates. Treatment of neuromycoses require prolonged therapy with antifungal drugs at low doses to avoid side effects and overcome the effect of the blood-brain barrier.
- Some protist infections of the nervous systems are fatal if not treated, including primary amoebic meningitis, granulomatous amoebic encephalitis, human African trypanosomiasis, and neurotoxoplasmosis.
- The various forms of ameobic encephalitis caused by the different amoebic infections are typically fatal even with treatment, but they are rare.
- African trypanosomiasis is a serious but treatable disease endemic to two distinct regions in sub-Saharan Africa caused by the insect-borne hemoflagellate Trypanosoma brucei.
- Neurocysticercosis is treated using antihelminthic drugs or surgery to remove the large cysts from the CNS.
Multiple Choice
Fill in the Blank
Short Answer
- Why do nervous system infections by fungi require such long treatment times?
- Briefly describe how humans are infected by Naegleria fowleri.
- Briefly describe how humans can develop neurocysticercosis.
Critical Thinking
- Fungal meningoencephalitis is often the ultimate cause of death for AIDS patients. What factors make these infections more problematic than those of bacterial origin?
- Compare East African trypanosomiasis with West African trypanosomiasis.
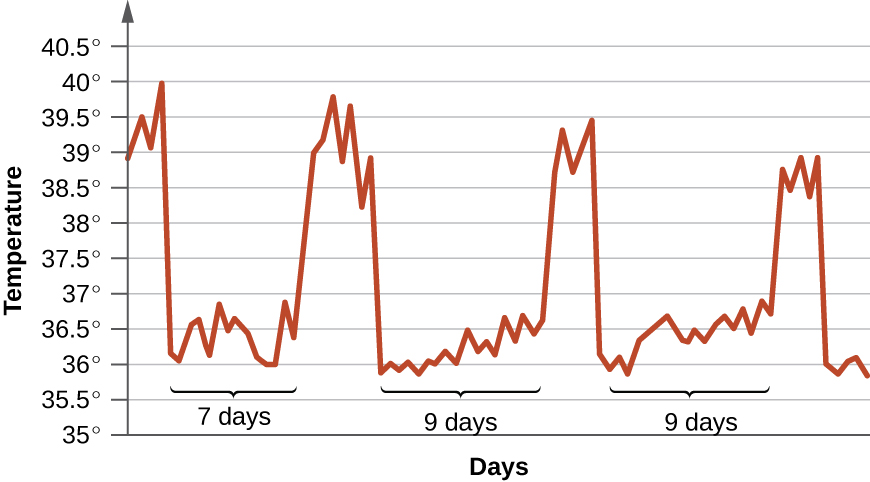
- The graph below tracks the body temperature of a patient infected with Trypanosoma brucei. How would you describe this pattern, and why does it occur?
Media Attributions
- OSC_Microbio_26_04_Cryptococc
- OSC_Microbio_26_04_Naegleria
- OSC_Microbio_26_04_AmoeEnceph
- OSC_Microbio_26_04_Trypanosom
- OSC_Microbio_26_04_Neurotoxpl
- OSC_Microbio_26_04_ParaTBL
- OSC_Microbio_26_04_ArtConnect_img
- US Centers for Disease Control and Prevention, “Naegleria fowleri—Primary Amoebic Meningoencephalitis (PAM)—Amebic Encephalitis,” 2016. Accessed May 14, 2019. http://www.cdc.gov/parasites/naegleria/treatment.html. ↵
- Dorlo, Thomas PC, Manica Balasegaram, Jos H. Beijnen, and Peter J. de Vries, “Miltefosine: A Review of Its Pharmacology and Therapeutic Efficacy in the Treatment of Leishmaniasis,” Journal of Antimicrobial Chemotherapy 67, no. 11 (2012): 2576-97. ↵
- US Centers for Disease Control and Prevention, “Parasites – African Trypanosomiasis (also known as Sleeping Sickness), East African Trypanosomiasis FAQs,” 2012. Accessed May 14, 2019. http://www.cdc.gov/parasites/sleepingsickness/gen_info/faqs-east.html. ↵
- US Centers for Disease Control and Prevention, “Parasites – African Trypanosomiasis (also known as Sleeping Sickness), Epidemiology & Risk Factors,” 2012. Accessed May 14, 2019. http://www.cdc.gov/parasites/sleepingsickness/epi.html. ↵
- Carruthers, Vern B., and Yasuhiro Suzuki, “Effects of Toxoplasma gondii Infection on the Brain,” Schizophrenia Bulletin 33, no. 3 (2007): 745-51. ↵
- Uppal, Gulshan, “CNS Toxoplasmosis in HIV,” 2015. Accessed May 14, 2019. http://emedicine.medscape.com/article/1167298-overview#a3. ↵
- Konradt, Christoph, Norikiyo Ueno, David A. Christian, Jonathan H. Delong, Gretchen Harms Pritchard, Jasmin Herz, David J. Bzik et al., “Endothelial Cells Are a Replicative Niche for Entry of Toxoplasma gondii to the Central Nervous System,” Nature Microbiology 1 (2016): 16001. ↵

