Protocol for Bacterial Cell Inhibition Assay
Please click this hyperlink to access the virtual lab bench

|
Materials and Reagents
|
|
| Reagents: | Materials: |
|
|
1. The final concentration of antibiotic for each well is shown below. You will be adding a set volume of antibiotic per well (20 µL), along with 180 µL of bacterial cell culture for a total volume of 200 µL in each well. Your stock concentration of antibiotic is 250 mg/mL. This is a very concentrated stock! This means that you need to first make working stocks for each final concentration of antibiotic. Prior to the lab, calculate how you will prepare the antibiotic working stocks to a final volume of 200 µL for each final concentration of antibiotic. You already have the working stock for your 25 mg/mL final concentration of antibiotic.
2. Please use serial dilutions to make the remaining 6 working stocks (G-B). This means that you will use sample H working stock as your stock solution to make sample G. You will then use sample G as your stock to make sample F, etc. Please note that the sample designation corresponds to the row number on your 96-well plate. Your INSTRUCTOR will double check your numbers before you start on the lab.
3. Table 1: Preparation of Antibiotic stock solution:
|
Sample (aka. row on the plate) |
Final concentration of antibiotic/well |
Working stock concentration (mg/mL) |
Calculate how to make the working stock concentrations (200 µL final volume) |
|
B |
5 µg/mL |
0.05 |
|
|
C |
50 µg/mL |
0.5 |
|
|
D |
500 µg/mL |
5 |
|
|
E |
1 mg/mL |
10 |
|
|
F |
5 mg/mL |
50 |
|
|
G |
10 mg/mL |
100 |
|
|
H |
25 mg/mL |
250 |
Already provided |
Step 4 will be conducted for you by the kind folks in the Biochemistry teaching labs
4. Cultures of E. coli strain 1 and 2 will be grown overnight to saturation.
5. Cultures will be diluted to an OD600 of 0.1 using LB. Your lab instructor will show you how to do this.
6. Ensure that your 96-well plate is clearly labelled on the side of the plate, not on the lid, before adding anything to the plate. This includes your initials, date, instructor name, lab day, contents of the plate AND any safety considerations.
7. Prepare 200 µL of each Antibiotic working stock solution by serial dilution (Table 1). You will be given 200 µL of 250 mg/mL Antibiotic.
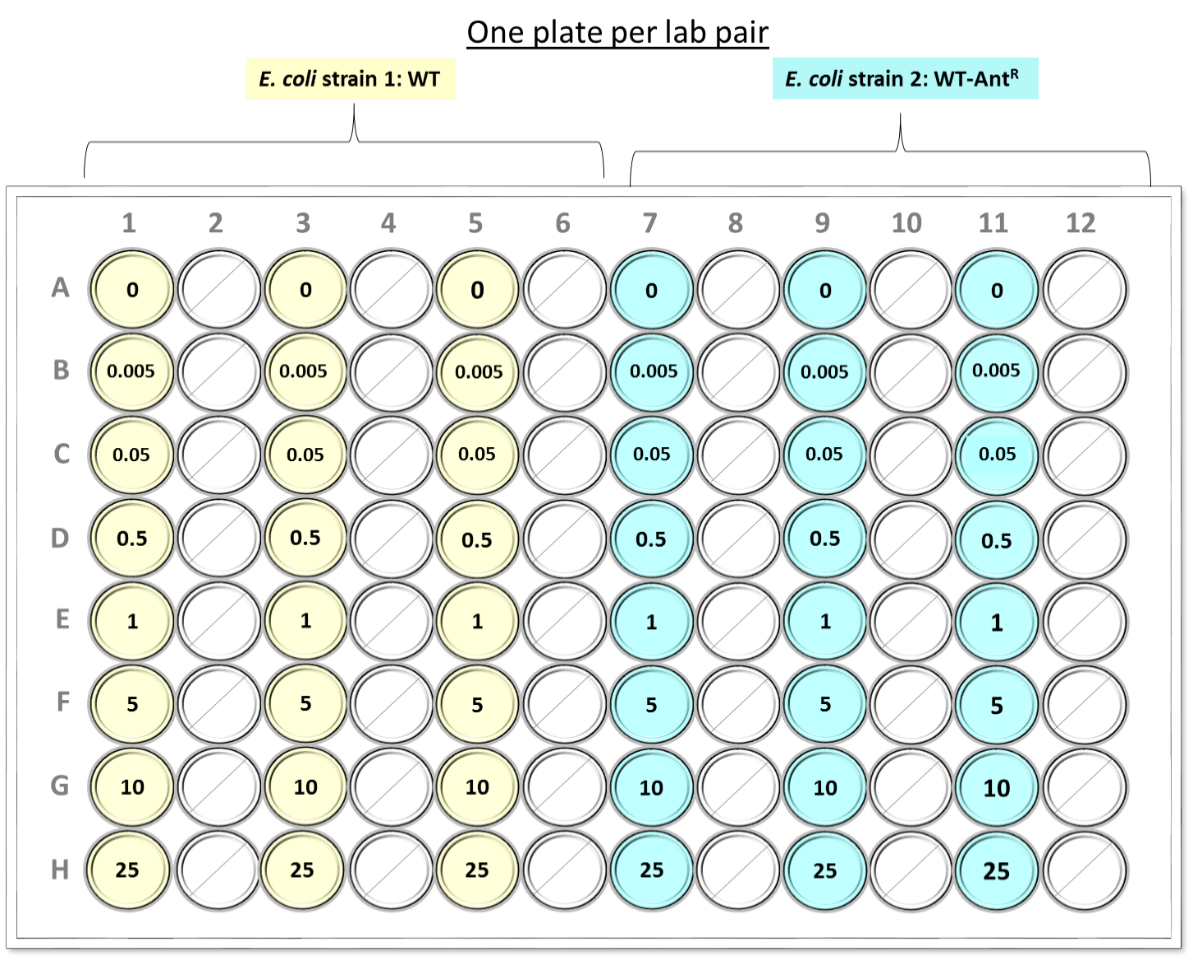
The table and schematic diagram below outlines the cultures and antibiotic concentrations that you will be adding to each well.
|
Lab pair – Plate Setup |
|||||
|
E. coli strain 1: WT |
E. coli strain 2: WT-AntR |
||||
|
control reactions (-ANT) * |
Wells A1, A3, A5 |
o |
control reactions (-ANT) * |
Wells A7, A9, A11 |
|
|
+ ANT (0.005 mg/mL) ** |
Wells B1, B3, B5 |
o |
+ ANT (0.005 mg/mL) ** |
Wells B7, B9, B11 |
|
|
+ ANT (stock 0.05 mg/mL) ** |
Wells C1, C3, C5 |
o |
+ ANT (stock 0.05 mg/mL) ** |
Wells C7, C9, C11 |
|
|
+ ANT (stock 0.5 mg/mL)** |
Wells D1, D3, D5 |
o |
+ ANT (stock 0.5 mg/mL)** |
Wells D7, D9, D11 |
|
|
+ ANT (stock 1 mg/mL)** |
Wells E1, E3, E5 |
o |
+ ANT (stock 1 mg/mL)** |
Wells E7, E9, E11 |
|
|
+ ANT (stock 5 mg/mL) ** |
Wells F1, F3, F5 |
o |
+ ANT (stock 5 mg/mL) ** |
Wells F7, F9, F11 |
|
|
+ ANT (stock 10 mg/mL) ** |
Wells G1, G3, G5 |
o |
+ ANT (stock 10 mg/mL) ** |
Wells G7, G9, G11 |
|
|
+ ANT (stock 25 mg/mL) ** |
Wells H1, H3, H5 |
o |
+ ANT (stock 25 mg/mL) ** |
Wells H7, H9, H11 |
|
|
* (–ANT wells) – Add 180 µL of the bacterial culture to each well. Add 20 µL of water to each well, in place of the antibiotic. ** (+ ANT wells) – Add 180 µL of the bacterial culture to each well. Add 20 µL of appropriate working antibiotic stock solution to each well. Please note that the antibiotic stocks refer to the final concentrations in the well *** (contamination control wells) – Add 200 µL of LB to each well. |
|||||
8. Incubate your plate in the 37°C incubator. Your plate will be read at the end of the day and again in the morning.
Once you are finished please wash your hands!

