Chapter 7 Lab Overview and Background Information
Learning Outcomes: Upon completion of this lab, students will gain practical experience in:
- Affinity column chromatography
- Pouring and running a column
Cell lysis
Before a protein can be purified it must first be extracted from the host organism. This is usually done by lysing the host organism. In this lab, we used bacteria as the host organism. Bacteria can be lysed by a variety of methods. One common way to lyse bacteria is through mechanical cell lysis. This can be done using a French press or a sonicator. A French press is an apparatus that lyses cells by passing them through a needle-thin valve under high pressure. Once the cells pass through the valve the pressure drops, this creates shear stress that disrupts the membrane. A sonicator emits high-frequency sounds waves that disrupt the membrane. Bacteria can also be lysed with the aid of a detergent. A detergent solubilizes the membrane lipid bilayer which subsequently leads to cell lysis. In this lab you will use a BugBuster protein extraction reagent from Novagen to lyse E. coli BL21(DE3) cells that over-expressed your DHFR-His-6x protein. BugBuster is detergent-based and Novagen asserts that BugBuster is “capable of cell wall perforation without denaturing soluble protein” (1).
In this lab you will purify your DHFR-His 6x tagged protein using affinity column chromatography.
Protein Purification: Ni-NTA Affinity Chromatography
Chromatography is a technique utilized in biotechnology to purify biological molecules such as proteins. The main goal of the technique is separation. Chromatography separates a complex mixture into individual, separate components.
Some basic chromatography terminology:
- Mobile phase – encompasses the solvent (usually a buffer) and the mixture of molecules requiring separation.
- Stationary phase – some form of matrix (such as the resin in a column) through which the mobile phase travels.
- Hardware – used to run a successful column chromatography experiment.
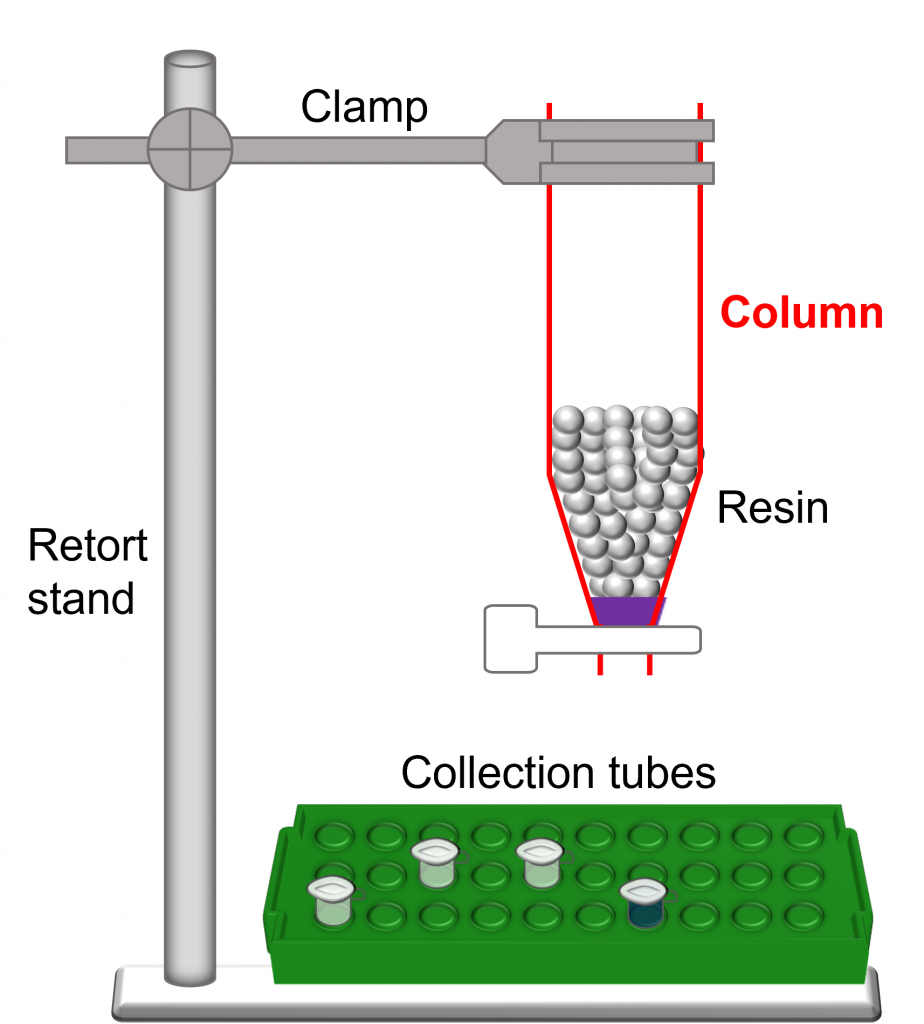
- Column bed (or resin bed) – mass/volume of resin/beads within a column.
- Void volume – volume of the space between beads. In essence, it is the volume of the mobile phase. For example, if the stationary phase occupies 45% of the total column volume, the void volume would be 55% of the total column volume.
- Exclusion limit – upper limit of a bead (or resin) type. This is the size above which proteins will elute in the void volume of the column.
Some Common Types of Chromatography:
•Gel filtration (or size exclusion) chromatography (2) – Gel filtration chromatography separates a mixture of molecules (let’s say proteins) based on size. The stationary phase usually contains microscopic beads (complete with a hole in them) which are packed into a column. When a mixture of molecules in a mobile phase is gently applied to the top of this column (very gently) the larger molecules quickly pass around the beads and make it to the bottom first. The smaller molecules enter the tiny holes in the beads and travel more slowly down the column. In this type of chromatography, the larger the molecule the faster it will travel down the column. The smaller the molecule the more time it needs to travel in and out of each hole in the beads. You can purchase different stationary phases that have varying hole sizes so you can choose whichever stationary phase (aka. resin) best fits your protein purification needs.
•Affinity chromatography (this is the type of chromatography you will be using in this lab)(3) – contains stationary phase (resin) that is coupled to something (an antibody, a metal, etc.) which binds your molecule/protein of interest. This is really cool because you can add your molecule/protein mixture to this resin and your protein of interest will bind to the stationary phase and thereby remain stationary. After washing the resin in the column you just need to elute (or un-bind) your protein/molecule of interest and voila: pure protein.
•Ion exchange chromatography (4) – this plays on the overall pI (isoelectric point) of the protein/molecule of interest you are trying to purify from a mixture. The resin can be either positively charged or negatively charged.
In this lab you are using affinity chromatography. To recap, affinity column chromatography is a separation technique in which your protein of interest can be isolated based on its specific interaction with a particular ligand that has been immobilized on a column matrix. You will be purifying two proteins from their respective cell lysates: DHFR-His-6x. The hexa-histidine (His 6x) tag has an affinity for nickel (5,6). This interaction can be manipulated by packing a column with an inert resin (let’s say agarose or sepharose) that has been pre-loaded with nickel. After adding your cell lysate to the column, you will wash away all other unwanted proteins that have not bound the column and elute your DHFR-His 6x proteins using one of a number of different elution techniques that release your histidine tags from the matrix. In your case this happens to be imidazole.
Workflow for purifying DHFR-His (6) protein using Ni-NTA affinity chromatography:

