Statements 5 to 10
Statement 5 – Debridement of wounds with adequate pain control when appropriate.
Debridement is one of the elements of the DIME [debridement of devitalized tissue, infection or inflammation control, moisture management and (wound) edge effect] principles of local wound care (Snyder et al., 2016). Debridement is the removal of dead tissue. It can also remove senescent cells, slough, debris, or any other extraneous materials, thereby promoting re-epithelization (Manna et al., 2021) . There are different types of debridement (Wound Debridement Options, 2018). Sharp surgical debridement for healable wounds is the most aggressive, using surgical instruments to remove dead or devitalized tissue to bleeding or viable wound base (creating an acute wound in a chronic wound). It requires adequate blood supply to ensure healing post debridement. The ability to perform acute sharp debridement is based on various factors including, each practitioner’s skill and scope of practice, availability of instruments, need for anesthesia, practice location and back up personnel.
Conservative surgical debridement consists of removing non-viable slough or dead tissue. This may be performed by removing surface slough but not cutting into live viable tissue of the wound base or edges (Sibbald et al., 2021). This type of debridement is often performed in maintenance or non-healable wounds. Other types of debridement are primarily performed on healable wounds and include enzymatic (enzymes chemically liquefy dead tissue), biological (e.g., maggot therapy), autolytic (using various dressings) and mechanical (hydrotherapy, wet-to-dry dressings that may cause more tissue damage more than the clinical benefit). See Figure 3.
Figure 3. Debridement methods for healable wounds
|
For Wounds That Can Heal |
||||
|
Debridement |
||||
|
Surgical |
Autolytic |
Biological |
Mechanical |
Chemical/Enzymatic |
|---|---|---|---|---|
|
|
|
|
|
Statement 6 – Assess and treat wounds for infection or inflammation.
Wound infection and inflammation comprise the “I” in the DIME (Debridement, Infection/Inflammation, Moisture Management, Edge Effect) approach. Wound infections can be superficial where there is critical colonization/local infection or be present in deep and surrounding compartment (Sibbald et al., 2006, 2017). Sibbald et al. uses an analogy of the wound as a soup bowl with a thin layer of soup to explain this concept. The superficial compartment that can be treated with topical antimicrobials, with the soup bowl representing the deep and surrounding tissue compartments requiring systemic antimicrobial treatment (Sibbald et al., 2017).
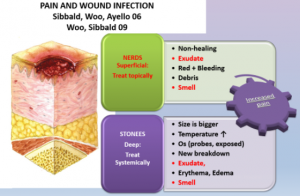
6a) Treat local infection topically. The NERDS and STONEES tool is a validated clinical tool to differentiate superficial from deep and surrounding infection (Woo & Sibbald, 2009). Any three or more NERDS criteria (Nonhealing, Exudate, Red friable tissue, Debris, Smell) indicate local infection and would need to be treated topically with appropriate antiseptics and antimicrobial dressings e.g., silver, iodine, polyhexamethylenebiguanide (PHMB)/chlorhexidine, Methylene Blue/ Gentian Violet.
6b) Treat deep and surrounding infection with systemic antimicrobials. Any three or more STONEES criteria [Size increase, Temperature differential of >3 oF on mirror image part of body, Os (probe to bone), New areas of breakdown, Exudate, Edema, Smell] indicate presence of deep and surrounding infection. Pain may be an additional third sign.
6c) Assess for presence of inflammation and using anti-inflammatory agents where necessary. Chronic wounds can also have an extensive and lengthy inflammatory response that impedes wound healing. There are various methods to diagnose wound Inflammation including measuring proteases [particularly matrix metalloproteinases (MMPs) and other biomarkers] however, they are not easily accessible in everyday practice (Saleh et al., 2019). It is necessary to consider the inflammatory properties of agents according to the wound status e.g., iodine is a proinflammatory antimicrobial, while silver is anti-inflammatory (Sibbald et al., 2021)
Statement 7 – Moisture management.
When selecting dressings, clinicians should aim for a comprehensive approach and consider patient satisfaction, improved wound outcome and cost of care (Brindle & Farmer, 2019).
7a) For healable wounds where the aim is to achieve moisture balance and facilitate autolytic debridement, dressings such as gelling fibres, calcium alginates, hydrogels, acrylics, hydrocolloids, and films are recommended for use.
7b) When only moisture balance is required, super absorbents, foams, calcium alginates, hydrocolloids, and hydrogels are used.
7c) Moisture reduction is needed for maintenance and nonhealable wounds. The goal of care is to decrease moisture and bacterial load.
Constant reevaluation of the state of the wound and patient would determine the choices of dressings and topical treatments that are appropriate at any given time. It is important to assess patient preference and not induce pain or discomfort. Dressings with PHMB/chlorhexidine and those with iodine can be active with decreased moisture and reduce bacterial load at the same time. Silver needs to be ionized for antibacterial action and this requires a moist wound interphase that is contraindicated in non-healable or maintenance wounds.
7d. Wound packing can also be done with wet (saline) packing (healable wounds) where tunneling is involved.
Table 4. Four ribbon gauze choices for wound packing
| Type ribbon gauze | Action | Comment |
|---|---|---|
| Saline soaked | Donate moisture to wound | Not antibacterial |
| Plain – dry | Absorb exudate from the wound | Not antibacterial |
| PHMB (AMD Gauze) PolyHexaMethyleneBiquanide |
Kills bacteria entering the gauze | Non-release = neutral to wound surface |
| Povidone Iodine-soaked gauze | Releases iodine to kill bacterial on the wound surface including biofilms | -Use with caution with thyroid disease -Pro-inflammatory to the wound surface |
Statement 8 – Evaluate the rate of healing.
8a) Reassess wounds that are stalled (healable) for other causes and diagnosis. A comprehensive assessment is necessary to further investigate other wound diagnoses. One way to identify a wound that needs to be reassessed is to calculate the reduction in wound volume between week 0 and week 4. If a wound is not 20-40% smaller by week 4, the wound is unlikely to heal by week 12. Often, a wound biopsy and a referral to an interprofessional assessment team would revise the diagnosis and treatment to try and establish an enhanced wound trajectory.
Statement 9 – Edge effect
9a) These modalities should be chosen based on evidence for their use and applicability to the individual patient, after a comprehensive, interprofessional assessment has been completed (Sibbald et al., 2021).
9b) Skin grafts have been successfully used in some cases, especially for deep and extensive thermal burns with recent studies detailing the use of natural skin substitutes that are bioengineered including acellular in vitro cultured skin cells (Łabuś et al., 2021). Adjunctive therapies are used for wounds that are stalled but healable. These modalities include: ultrasound, negative pressure wound therapy (NPWT), electrical stimulation, hyperbaric oxygen (HBO), dermal substitutes/skin grafts, platelet-enriched plasma and reconstructive surgery (Boersema et al., 2021).
Statement 10 – Organizational support.
Organizations, their governing principles and current practices should enable evidence informed practices, interprofessional education and team work, multidirectional communication, quality improvement programs and patient-centered care (Engle et al., 2021; Sibbald et al., 2021). The Porter Model of health care that connects 6 other P’s but most importantly starts with the patient and includes the (healthcare) professional, provider, payer, policy maker and politician. This model provides value for the health care dollar (Adamkasi, 2017; Sibbald et al., 2021).

