Skin Grafting and CTPs
Skin Grafting
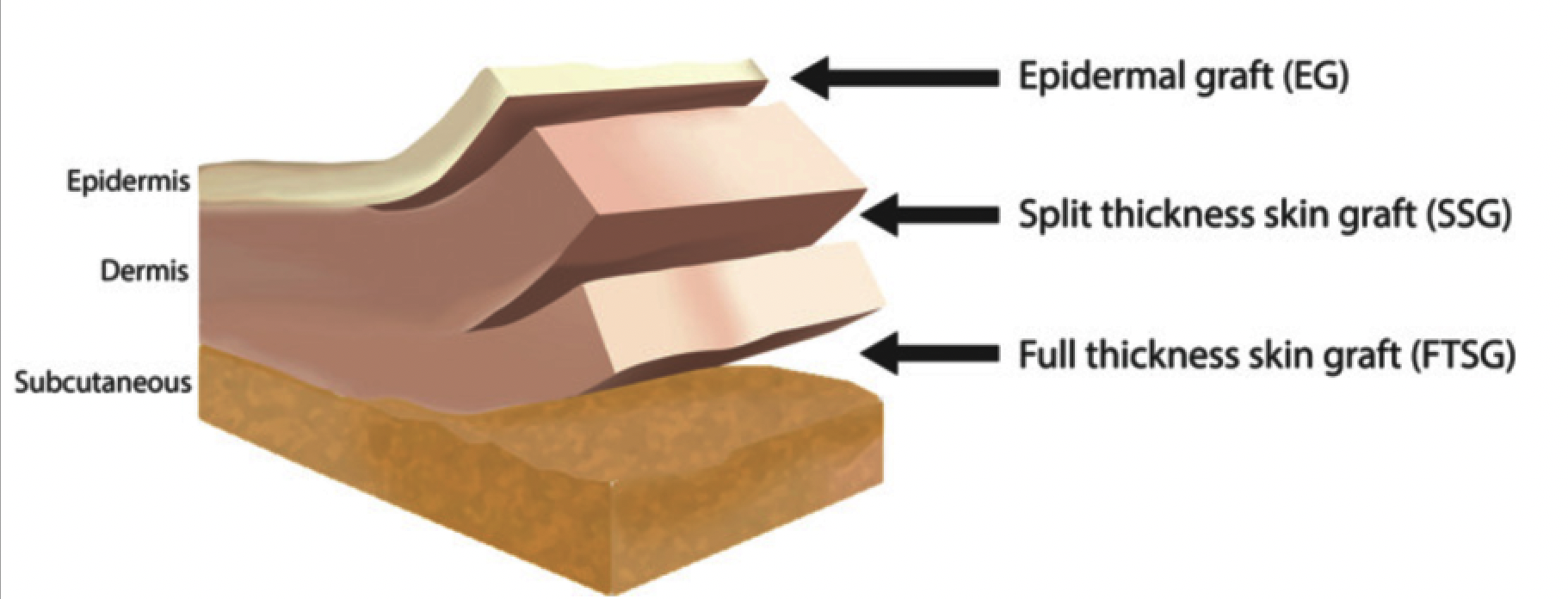
Skin grafting, where skin or a skin substitute is transplanted over a wound, may be a useful and effective adjunctive therapy for stalled healable wounds (Serra et al. 2017). A skin graft provides the wound with the keratinocytes needed for epithelization along with mechanical stability and resistance to infection and fluid loss (Hierner et al. 2005). Skin grafts are indicated in a variety of clinical situations, such as chronic, traumatic and burn wounds (Elseth and Lopez 2021; Serra et al. 2017). Classification of skin grafts is based on the thickness of the harvested skin: full‐thickness skin graft (FTSG), split‐thickness skin graft (SSG) and epidermal graft (EG) (Figure 3; (Kanapathy et al. 2017)). FTSG offers the best cosmetic outcome, while SSGs are frequently used for functional repair in wounds such as venous or arterial ulcers as they require less blood supply. Evidence suggests high success rates with skin grafts in chronic venous leg ulcers (VLUs) compared to chronic arterial ulcers and DFUs (Serra et al. 2017).
Figure 3: Skin Grafts Depth (Kanapathy et al. 2017)
Both FTSG and SSG require hospital admission, a trained surgeon, operating room and anaesthesia. They are associated with a significant burden to the donor by creating a painful wound at the harvesting site (Herskovitz et al. 2016). In contrast, EG can be done in an outpatient setting without donor site morbidity. EG also stimulates wound healing differently from FTSG and SSG, likely through providing the wound with growth factors and cytokines (Herskovitz et al. 2016). A systematic review concluded that EG is associated with a wound healing rate of over 70% and a mean healing time of 5 weeks (Kanapathy et al. 2017). The healing rate varied by wound etiology; DFUs, burn wounds, and SSG donor site wounds achieved complete healing with EG, while conditions including lymphedema and pyoderma granulosum, failed to heal with EG. Finally, it is imperative to optimize the recipient wound bed to avoid skin grafts failure (Herskovitz et al. 2016).
Cellular and/or tissue-based products (CTPs)
Previously developed to treat burns and traumatic wounds, CTPs may also improve wound healing in DFUs and VLUs (Liu et al. 2019). The synthetic material of skin substitutes mimics normal skin, providing a moisture coverage and a bacterial barrier. CTPs help replace lost tissue to restore normal function by providing a template for host’s cells to use for healing (Ho et al. 2005). Different types of skin substitutes are available with varying wound indications, effectiveness in diverse chronic wounds and evidence for their use (Nicholas and Yeung 2017). For example, acellular dermal substitutes involve materials similar to the extracellular matrix present in skin that provide a scaffold for wound healing without causing immune reactions (Abdo and Ortman 2020). Their effect has been studied on DFUs and showed shorter healing times (Nicholas and Yeung 2017). On the other hand, cellular dermal substitutes include fibroblasts and/or keratinocytes that secrete cytokines and growth factors to replace tissue loss but are at risk for immunogenicity (Abdo and Ortman 2020; Nicholas and Yeung 2017). To overcome this, cells from amniotic membrane and neonatal foreskin have been employed to avoid immune rejections. Cellular-based CTPs have been proven to promote wound healing in both DFUs and VLUs and may potentially offer economic net savings (Liu et al. 2019). In the upcoming years, CTPs will involve the development of an ideal skin substitute (Figure 4) as well as the replacement of other skin components, such as hair follicles and sebaceous glands (Liu et al. 2019; Nicholas and Yeung 2017).
Figure 4: Ideal properties for skin substitutes ( Shores et al., 2007)