16. REDOX and Electrochemistry Problems
Redox and Electrochemistry Problems
1. For the following reactions in acidic aqueous solution, write the balanced half reactions for the oxidation and the reductions and balance the full reaction.
- Fe2+ + O2 → Fe3+.
- H2O2 + Sn2+ → Sn4+ + H2O
- H2O2 → H2O + O2
- SbCl4– + SO42– + Cl– → SbCl6– + H2SO3
- PbS + H2O2 → PbSO4 + H2O
- Mn2+ + BiO3– → N2O4
- NO + NO 3– → N2O4
- CS(NH2)2 + BrO3– → CO(NH2)2 + SO42–
2. For the following reactions in basic solution, write the balanced half reactions and the balance the overall reaction.
- C2H4 + MnO4– → CH3COO– + MnO2
- ClO– → ClO3– + Cl–
- Al(s) + NO3– → Al(OH)4– + NH3
- Bi(OH)3 + Sn(OH)42– + NH3
- MnO4– + CN– → MnO2 + OH–
- S + HO2– → SO42– + OH–
3. Determine the oxidation number of Phosphorus in each of the following: H3PO4, HPO32–, PH3, PCl+, PCl5, PCl6–.
4. Determine the oxidation number of chromium in each of the following: Cr2O7– , Cr2O42–, CrOCl–, Cr2Cl93–.
5. What mass of zinc metal is deposited in an electrolytic cell containing Zn2+ if a current of 100. amperes is passed through it for a period of 10. hours? [1.22 kg]
6. Determine the oxidation number of S in Sodium thiosulfate and in sodium tetrathionate, Na2S2O3, Na2S4O6.
7. What is the oxidation number of Carbon in the following?
CH3Cl, HCOOH, CCl2F2, CH3NH2.
8. A sample of sodium of mass 3.460 g is reacted with air and produces solid sodium oxide. What is the balanced chemical equation for the process? The oxide is dissolved in water to make an acidic solution. What is the reaction for this process?
9. Write the cell reactions for the following and calculate their standard cell potential ![]() :
:
- Cu(s) | Cu2+(aq) || Zn2+(aq) | Zn(s)
- Cd(s) | Cd2+(aq) || Fe3+(aq) , Fe2+(aq) | Pt(s)
- Pt(s) | Cl¯(aq), Cl2(g) || HClO(aq), Cl2(g) | Pt(s)
[a -1.1047 V, b +1.174 V, c +0.252 V]
10. Balance each of the following redox equations in aqueous solution and determine the standard cell potentials ![]() .
.
- NO3¯(aq) + Cu(s) → Cu2+(aq) + NO(g) [+0.6115 V]
- I2(s) + S2O32¯(aq) → I¯(aq) + S4O62¯(aq) [+0.46 V]
- H2SO3(aq) + IO3¯(aq) → SO4 2¯(aq) + I2(s) [+1.023 V]
11. Write the cell reactions for the following cells. Determine the cell potential and identify the anode and the positive electrode in each case.
- Pt(s) | Fe3+(aq), Fe2+(aq) || Cl2(g), Cl¯ | Pt(s) [+0.587 V]
- Fe(s) | Fe2+(aq) || O2(g), H+(aq), H2O(
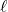 ) | Pt(s) [+1.676 V]
) | Pt(s) [+1.676 V] - Fe(s) | Fe2+(aq) || Zn2+(aq) | Zn(s) [–0.316 V]
- Pt(s) | H2(g), HCl(aq) || AgCl(s) | Ag(s) [+0.22233 V]
12. Calculate the cell potential for the following cell. Note that it’s not at standard state.
Pt(s) | H2(g,100 kPa), HCl(aq, 0.01 M) || AgCl(s) | Ag(s)
13. Consider the following cell: Ag(s) | AgBr(s) | NaBr(aq, 1.0 M) || AgNO3(aq, 1.0 M) | Ag(s)
The cell potential of the cell is +0.728 V, i.e., the right-hand electrode is at a positive potential of 0.728 V, relative to the left-hand electrode. Calculate the solubility product constant of silver bromide, AgBr, and compare your result with the solubility tables. [4.9×10¯13]
14. Use the standard reduction potentials to calculate the solubility product constant of AgIO3.
[2.9×10¯8]
15. Calculate ![]() for the following half reaction from Gibbs Energy of formation values found in the Table of Thermodynamic values.
for the following half reaction from Gibbs Energy of formation values found in the Table of Thermodynamic values.
MnO2(s) + 2H+(aq) + 2e¯ → Mn2+(aq) + 2H2O(![]() ) [1.229 V]
) [1.229 V]
16. Use the following data
1/2H2O2(aq) + H+(aq) + e¯ → H2O(![]() )
) ![]() = +1.776 V
= +1.776 V
O2(g) + 4 H+(aq) + 4 e¯ → 2H2O(![]() )
) ![]() = +1.229 V
= +1.229 V
to determine the value of ![]() for the following half-reaction:
for the following half-reaction:
O2(g) + 2 H+(aq) + 2 e¯ → H2O2(aq)
[+0.682 V]
17. Calculate the value of ![]() and
and ![]() for the following reaction, using the data from the preceding two questions.
for the following reaction, using the data from the preceding two questions.
MnO2(s) + H2O2(aq) + 2 H+(aq) → Mn 2+(aq) + O2(g) + 2 H2O(![]() )
)
[+0.547 V, 3×1018]
18. Calculate ![]() for the reduction of Zn(OH)2(s) to zinc metal in basic solution at pH of 14 using the Ksp value in the solubility data table.
for the reduction of Zn(OH)2(s) to zinc metal in basic solution at pH of 14 using the Ksp value in the solubility data table.
Zn(OH)2(s) + 2 e¯ → Zn(s) + 2 OH¯(aq)
[-1.25 V]
19. Calculate ![]() for the commercial mercury cell using the thermodynamic data and determine which is the positive electrode. The cell can be represented using the following diagram.
for the commercial mercury cell using the thermodynamic data and determine which is the positive electrode. The cell can be represented using the following diagram.
Zn(s) | ZnO(s) | OH¯(aq) || HgO(s) | Hg(![]() )
)
[+1.35 V]
20. Balance the following equation for a disproportionation reaction using the smallest possible integer stoichiometric coefficients, and calculate the equilibrium constant for the reaction.
ClO3¯(aq) → ClO4¯(aq) + HClO2(aq)
[7.0]
21. A fuel cell uses methane, CH4(g) as a fuel and oxygen O2(g) as an oxidizer to produce electrical energy by means of the following reaction:
CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(![]() )
)
- write the balanced equations for the half-cell reactions.
- Using the Gibbs energy of formation from the thermodynamic data tables, calculate
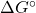 and
and 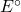 for the cell reaction at 25℃. []
for the cell reaction at 25℃. [] - In real usage situations, the cell potential is 1.0 V. How much electrical energy could be obtained per gram of methane?
[b -817.9 kJ/mol, + 1.06, c V48.9 kJ/g]
22. From the half-reactions for the electrolytic production of aluminum, calculate the theoretical mass of carbon that is oxidized at the anode per gram of aluminum metal produced.
[0.33 g C/g Al]
23. Consider the following cell: Pt | H2(g, 100 kPa), H+(aq, 1.0 M) || Cu2+(aq) | Cu(s). The left-hand electrode is the standard hydrogen electrode (SHE).
- If
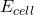 = +0.20 V, what is the concentration of Cu2+(aq)?
= +0.20 V, what is the concentration of Cu2+(aq)? - If NaOH is added to the solution at the right-hand electrode, and Cu(OH)2(s) precipitates, calculate
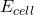 when [OH¯] = 0.50 M, if the solubility product constant for Cu(OH)2 is 1×10¯20.
when [OH¯] = 0.50 M, if the solubility product constant for Cu(OH)2 is 1×10¯20.
[a. 1.6×10¯5 M, b. -0.212 V]
24. A silver rod is immersed in an aqueous solution saturated with silver oxalate Ag2C2O4. The solution is connected by a salt bridge to a standard hydrogen electrode to form a galvanic cell in which the silver rod is at a potential of 0.589 V relative to the hydrogen electrode.
- Write a balanced half-cell reaction to describe the processes at each electrode.
- Write the full cell reaction
- Calculate the solubility product constant for silver oxalate.
[1×10¯11]
25. The following electrochemical cell is set up:
Cu(s) | Cu2+(aq, 0.100 M) || Cu2+(aq, 0.0100 M) | Cu(s)
- Calculate the cell potential and determine which electrode is positive.
- 1.0 mL of a 5.0 M aqueous solution of ammonia NH3 is added to the solution at the left-hand electrode, which has a volume of 100. mL. Calculate the new cell potential.
- What is the cell potential if a total of 100. mL of the ammonia solution is added to the solution at the left-hand electrode? The equilibrium constant for the following reaction:
Cu2+(aq) + 4 NH3(aq) Cu(NH3)42+ is 4.3×10+12.
Cu(NH3)42+ is 4.3×10+12.
[a -0.030 V, b -0.028 V, c +0.40 V]

