5. Thermodynamic I questions
Michael Mombourquette
- If the standard enthalpy change
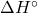 for the reaction
for the reaction
SO2(g) + ½ O2(g) → SO3(g) is -98.9 kJ/mol, what is the standard enthalpy change for each of the following reactions?- 2 SO2(g) + O2(g) → 2 SO3(g) [-197.8 kJ/mol]
- SO3(g) → SO2(g) + 1/2 O2(g) [+98.9 kJ/mol]
- Calculate
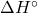 for the dehydrogenation of ethane,
for the dehydrogenation of ethane,
C2H6(g) → C2H4(g) + H2(g), form the following data: [+136.9 kJ/mol]C2H4(g) + 3 O2(g) → 2 CO2(g) + 2 H2O( )
) 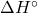 = -1411.0 kJ/mol
= -1411.0 kJ/mol
C2H6 + 3½ O2(g) → 2 CO2(g) + 3 H2O(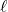 )
) 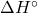 = -1559.9 kJ/mol
= -1559.9 kJ/mol
H2(g) + ½ O2(g) → H2O(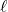 )
) 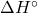 = -285.8 kJ/mol.
= -285.8 kJ/mol. - Calculate
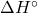 at 25℃ for the combustion of buckminsterfullerene, C60(s) per mole of carbon atoms and compare the result for that of graphite, C(s, graphite). [-432.3 kJ/mol]
at 25℃ for the combustion of buckminsterfullerene, C60(s) per mole of carbon atoms and compare the result for that of graphite, C(s, graphite). [-432.3 kJ/mol] - Stearin, with formula C57H110O6, is a typical fat and its oxidation is an important source of energy in the body. The standard enthalpy change of combustion of stearin in oxygen to give carbon dioxide and liquid water is -37.7 MJ/mol. write a balanced equation for the combustion reaction and calculate the standard enthalpy change of formation of stearin.
- Use data from the thermochemical tables to calculate
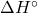 per mole for each of the following processes at 25℃.
per mole for each of the following processes at 25℃.
- CaCO3(s) → CaO(s) + CO2(g) [+178.32 kJ/mol]
- CaO(s) + H2O(
 ) → Ca(OH)2(s) [-65.17 kJ/mol]
) → Ca(OH)2(s) [-65.17 kJ/mol]
- Use data from the thermochemical tables to calculate
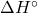 for each of the following reactions at 25℃.
for each of the following reactions at 25℃.
- CH4(g) + ½ O2(g) → CH3OH(
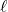 ) [-163.85 kJ/mol]
) [-163.85 kJ/mol] - CH4(g) Cl2(g) → CH3Cl(g) + HCl(g) [-98.33 kJ/mol]
- CH4(g) + ½ O2(g) → CH3OH(
- Calculate the heat of combustion per gram of ethane, C2H6(g), and propane, C3H8, in air at 25ºC. Use Formation enthalpies to do this. Compare with the table of combustion energies found in the chapter.
- Calculate the standard enthalpy change at 25℃ for dissociation of each of the following gas molecules into atoms: H2, F2, Cl2, Br2, I2, O2, N2. Note, in standard state, Br2 is a liquid and I2 is a solid.
[+435.9, +158.0, 243.4, 192.9, 151.2, 498.3, 945.4 kJ/mol] - Calculate the standard enthalpy change at 25℃ for the dissociation of the following molecules into atoms: HF, HCl, NO.
[569.1, 432.0, 631.6 kJ/mol] - Calculate the enthalpy change at 25℃ for the neutralization of acetic acid by sodium hydroxide in aqueous solution as described by the net ionic equation
CH3COOH(aq) + OH–(aq) → CH3COO–(aq) + H2O(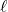 ). [-56.09 kJ/mol]
). [-56.09 kJ/mol] - Calculate the enthalpy change at 25℃ for the neutralization of aqueous ammonia NH3 (aq), a weak base, with hydrochloric acid. The net ionic equation is NH3(aq) + H+ → NH4+(aq). [-52.22 kJ/mol]
- Calculate the enthalpy change at 25℃ for the reaction between SO2(g) and H2S(g) to give elemental sulfur, a reaction used to purify ‘sour’ gas.
SO2(g) + 2 H2S(g) → 3 S(s) + 2 H2O(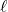 ). [-233.57 kJ/mol]
). [-233.57 kJ/mol]

