Appendix 7 Table of solubilities
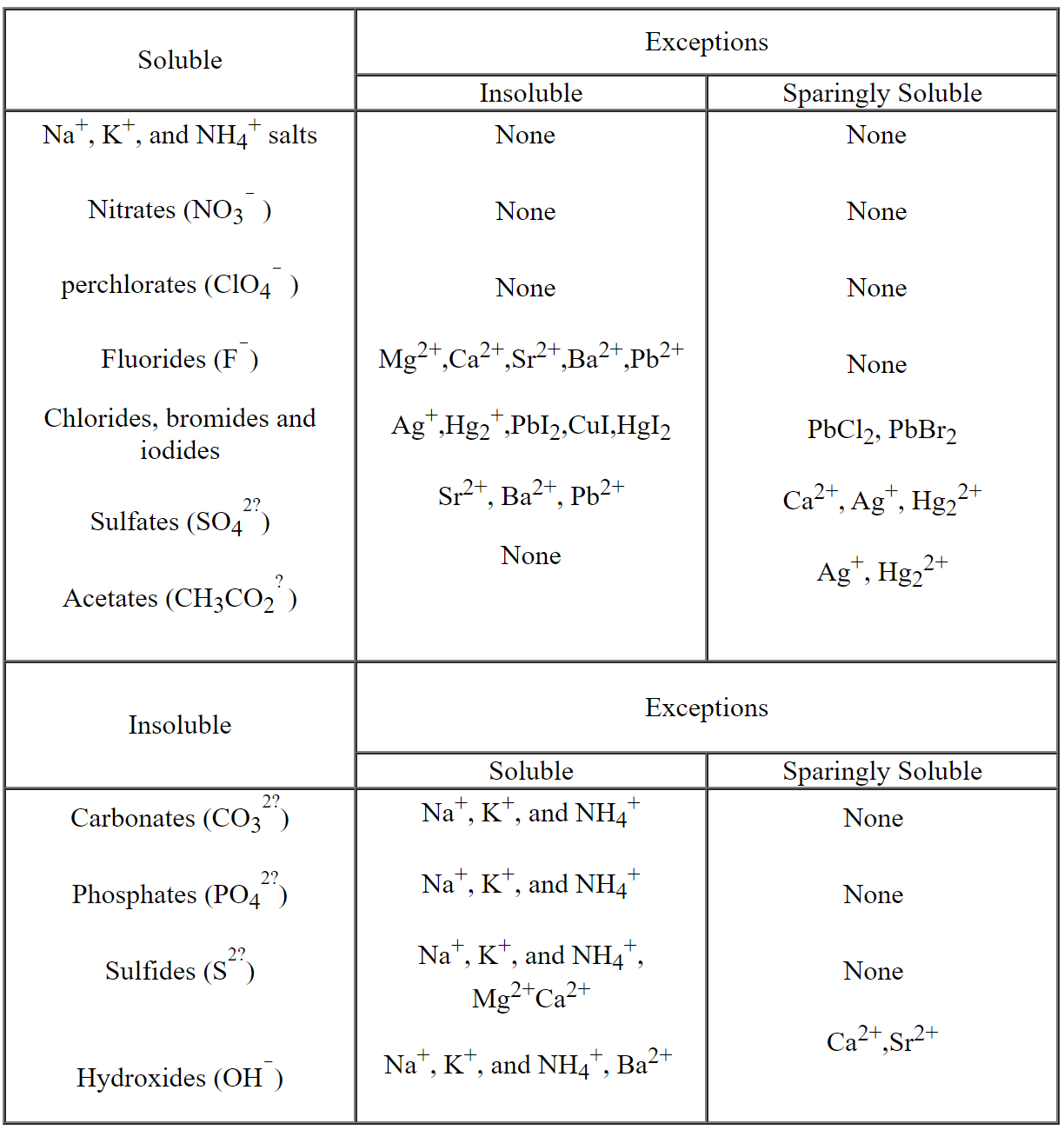
Table of Solubilities: This table gives us a quick guide to help
determine whether a particular compound is soluble, partially soluble or
insoluble.
Solubility Product Constants
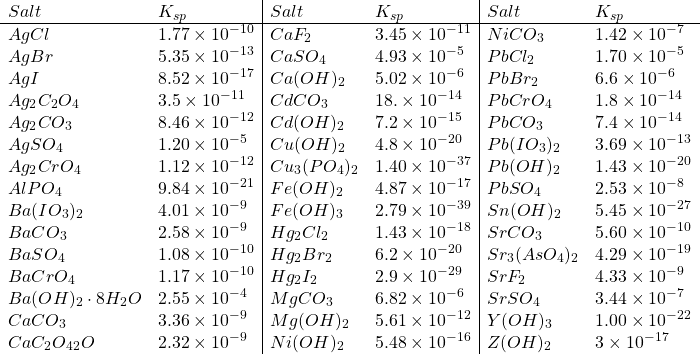
Table of Solubilities: This table gives us a quick guide to help
determine whether a particular compound is soluble, partially soluble or
insoluble.

Solubility Product Constants
