Glossary
Michael Mombourquette
- Activity
- The activity is a unit-independent measure of the availability of an individual component to become involved in a chemical reaction. It is a fairly advanced thermodynamic concept, which you will see in more detail in upper-year Phys. Chem. courses. In first year, we will use a simplified version called the relative activity of a substance. This is a unitless parameter that we derive by dividing the measure (concentration or pressure) by the standard measure of that substance. For example, the standard pressure of an ideal gas is 1 bar or 100 kPa. Thus, to convert a measured pressure of a gas into its activity, we divide the measured pressure by the standard pressure. Thus, if a gas has a measured pressure of, say, 54 kPa, it will have a relative activity of 54/100 = 0.54.
The standard concentration of a solute in solution is 1 mol/L. Thus, a solute with a concentration of 0.32 M would have a relative activity of just 0.32. - Algebraic Method
- The method of
balancing chemical equations that involves solving simultaneous linear equations using algebra. Coefficients are assigned to the chemicals and the sum of coefficient on the reactant side must equal the sum of coefficients on the product side for each element and for the charge. This gives a series of simultaneous linear equations that can be solved algebraically. The method is described HERE. - Amphiprotic
- A species (molecule or ion) that can act as a Brønsted-Lowry acid or base is called an amphiprotic species. In simpler terms, an amphiprotic species can donate a proton in some reactions and it can accept a proton in other reactions. The most familiar amphiprotic species is water. Water acts as an acid (donates a proton) when it reacts with a base and it acts as a base (accepts a proton) when it reacts with an acid. Other amphiprotic species are often found as the partially deprotonated form of poly-protic acids. For example, if one proton is removed from phosphoric acid H3PO4(aq)
we get H2PO4–(aq). This latter species can accept a proton to return to phosphoric acid or it can donate a proton to become HPO42-(aq). Thus, H2PO4–(aq) is amphiprotic. By similar logic, HPO42-(aq) is also amphiprotic. - Avagadro’s Constant (or number)
- The number of particles in one mole of particles (see mole). Measured to be NA = 6.0221×10-23
- Azeotrope
- An azeotropic solution (a.k.a. azeotrope) is one for which the liquid and vapour compositions are in equilibrium with each other are the same. The point on the boiling point versus composition curve in the two-component diagram is called the azeotrope. Azeotropes can occur at a maximum or a minimum in the curve, depending on the relative strength of the intermolecular forces involved. Compared to the individual liquids, lower solution intermolecular forces means the solution boils easier so we get a minimum-boiling azeotrope and higher solution intermolecular forces means a maximum-boiling azeotrope.
- Bonds
- Chemical Bonds are formed when two or more atoms are attracted to each other remain connected to each other in an energetically favorable (lower system energy) situation. The bonds can be thought of as consisting of coulombic interactions. Coulombs Law tells us that the force between two charges is given as
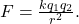 The forces involved are both attractive (if the two charges, q1 and q2 have opposite signs) or
The forces involved are both attractive (if the two charges, q1 and q2 have opposite signs) or
repulsive (if the two charges have the same signs). A stable bond is formed if a position can be found where the attractive and repulsive forces balance each other and where the resulting positions of the atoms (their positive nuclei and negative electrons) result in a lower energy state. - Brønsted-Lowrey Acid Base
- A Brønsted-Lowrey acid is a species that gives up (donates) a proton, when it reacts with a Brønsted Lowrey base. By corollary, the Brønsted-Lowrey base is a species that accepts a proton when it reacts with a Brønsted-Lowrey acid. The term acid and base are relative ones. An acid cannot exist without a base and vice versa. They are defined based in their interaction with each other.
- Calorimeter
- Device used in Calorimetry to measure heat processes (normally thermally insulated from the surroundings)
- Calorimetry
- Study of heat absorbed or evolved in chemical reactions
- Closed System
- Any system that is sealed but not insulated, for example, an aluminum water bottle will not lose water but it will get warm as the heat from the day is absorbed into the bottle.
- Thus, Heat may transfer into or out of the system but no material can transfer.
- Combustion Reaction
- This is a named reaction type. Generally, a combustion reaction for a certain compound involves the complete combustion in oxygen of one mole of said compound to it’s fully oxidized products. If the compound in question is a carbohydrate, the products are carbon dioxide and water. If there are other elements in the compound, the products would have to include the fully oxidized form of those elements. For example, the combustion reaction for ethane, C2H6 is
- C2H6 + 3.5 O2 → 2 CO2 + 3 H2O.
Note: the coefficient for the ethane MUST be 1 for it to be called the combustion reaction. The following balanced equation shows the same chemical process but is not the defined combustion equation. - 2 C2H6 + 7 O2 → 4 CO2 + 6 H2O.
- The combustion reaction for ethylamine has to have NO2 as a product in addition to carbon dioxide and water.
- CH3NH2 + 3.25 O2 → CO2 + NO2 + 2.5 H2O
- Conjugate Acid and Base
- When a species donates a proton (acts as a Brønsted-Lowrey acid), it leaves behind a deprotonated species, which might be able to accept back that proton (act as a Brønsted-Lowrey base). These two species are different only in the loss or addition of one proton. They are conjugate to each other. For example, ammonia (NH3) is the conjugate base of the ammonium ion (NH4+). Conversely, we might say that the ammonium ion is the conjugate acid of ammonia. The difference between ammonia and ammonium is one proton.
- Core
- The core of an atom consists of the nucleus of the atom and the electrons in all but the outermost principle energy levels. A simple model of the bonding in molecules considers the atom to be made up of the core plus the valence electrons, where only the valence electrons are involved in the actual bond and the core can always be considered as simply a point with a charge called the core charge.
- Core Charge
- The core charge is simply the sum of the charge numbers of the nucleus and electrons in the core of the atom. The core charge is defined as the atomic number, Z, minus the number of core electrons. The core charge can be calculated for main group elements simply by equating the core charge to the main-group column number, (excluding the transition element, etc, from the column numbering.)
- Covalent Bond
- A covalent bond is defined as a bond that is formed when a pair of electrons is shared between two atoms. In theory, the two electrons are equally shared between the two atoms. However, other considerations make that a rare occurrence in real molecules, where most ‘covalent’ bonds do not equally share the electron pair. We call a bond 100% covalent if the electron pair is exactly equally shared. This type of bond only occurs in homo-nuclear diatomic molecules. Many molecules are still considered to be
covalently bonded, even if the electron-pair sharing is not perfectly equal. - Covalent Solids
- Covalent (network) solids are made of atoms that are covalently bonded together to form one continuous network of covalently bonded atoms. One could almost think of this type of solids as macroscopic molecules (big enough to see).
- Daulton’s Law of partial pressures
- Daulton postulated that for ideal gases, ratios of moles of the gases is equal to ratios of the pressures of the gases. Thus, for a mixture of two gases A and B, the relative pressure of A versus B equals the relative amount of moles of A, compared to B.
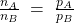
- Empirical Formula
- The chemical formula for a substance where the lowest whole-number ratio of the atoms in the substance is shown explicitly. for example, benzene has a carbon to hydrogen mole ratio of 1:1, even though there are six carbons and six hydrogens in a benzene molecule. The empirical formula for benzene is CH. Generally, we do not use the empirical formula to represent such chemicals and must use the molecular formula.
- End Point
- During a titration, the experimenter often adds an indicator to the solution. The indicator changes color when the correct conditions arise. The end point is simply the point during the experiment when the indicator changes color. The indicator is chosen such that the end point occurs at a meaningful point in the experiment. In an acid-base titration, the end point should occur just when the acid and base are exactly both used up (this is the equivalence point).
- Energy
- The capacity to do work or to transfer heat
- Endothermic
- Any process that absorbs heat is called an endothermic process. We generally see a process to be endothermic by observing the surroundings’ temperature drop. For example, a chemical in solution will react and absorb energy. If we define the chemicals as the system and the solvent as the surroundings, we will observe the temperature of the solvent (solution) go down during an endothermic process. This is the opposite of what we observe for an exothermic process.
- Enthalpy
- The enthalpy H is a measure of energy in contained within a system. Since we do not know the zero of enthalpy, we can never know the absolute enthalpy of any substance.
- Enthalpy change
- The enthalpy H is a measure of energy in contained within a system. As such, it is not a useful definition. We cannot measure the absolute enthalpy of a system but we can
measure the change in enthalpy for a process. This is defined as the amount of thermal energy transferred between a system and the surroundings under constant pressure. Enthalpy change is given the symbol ΔH. Enthalpy change is generally measured as a temperature change at constant pressure but can be determined by other means as well. Enthalpy change has SI units of Joules or kiloJoules. Unfortunately, the same symbol is often used for the molar enthalpy change (units of kJ/mol) of a chemical reaction. - Entropy
- The entropy (S) is a measure of the availability of microstates in a system. A microstate can be thought of as a combination of position and energy for all the particles in a system. Thus, the number of microstates that a system has available to it can depend on energy and on volume. The more energy a system has, the more high-energy microstates are available to be occupied. The more volume a system has, the more locationally different microstates a system has.
- Entropy Change
- The entropy change of a system process is defined as the total entropy of the final state minus the total entropy of the initial state as a result of the process itself. For example, a sample of pure liquid evaporates to the gas phase. We calculate the entropy change (ΔS) as simply the entropy of the gas minus the entropy of the liquid. More generally, we write
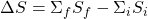
Where the summation occurs over all the components of the system in its final (f) and initial (i) states. - Equilibrium
- A dynamic equilibrium is defined as a system state whereby the forward rate is equal to the reverse rate. This applies to both chemical processes (reactants to products) and physical processes. Most of these processes can occur in both directions simultaneously, thus: reactants become products but simultaneously, products become
reactants, liquid evaporates to gas while gas condenses to liquid. These reverse direction phase changes that occur simultaneously lead to a system state that we call equilibrium when the rate of the forward process is the same as the rate of the reverse process. “Forward”, being defined by the way we’ve written the process down. Thus, in a chemical reaction, if the forward rate (reactants → products) is equal to the reverse rate (products → reactants) then there will be no net change in the amount of products or reactants. The apparent progress of the reaction, measured macroscopically will have stopped, even though at the microscopic level, the reaction continues. - Equilibrium constant
- See Thermodynamic Equilibrium Constant.
- Equivalence Point
- The equivalence point in an acid-base titration is the point in the experiment where the amount of acid (or base) from the burette is exactly equal to the amount of base (or acid) that was in the flask to start. At the equivalence point, the acid and base are exactly used up (neutralized) and only the conjugate ions of the original acid and base are left in solution.
- Exothermic
- Any process that gives off heat is called an exothermic process. We generally see a process to be exothermic by observing the surroundings’ temperature rise. For example, a chemical in solution will react and give off heat. If we define the chemicals as the system and the solvent as the surroundings, we will observe the temperature of the solvent (solution) go up during an exothermic process. This is the opposite of what we observe for an endothermic process.
- Extended Fraction
- The Extended Fraction formalism is a means of performing conversions of units and/or dimensions by expanding all the individual fractions in a problem to full explicit forms and then putting them into a single fractional form in a way that ensures the proper units cancel to leave the desired units of the answer. Thus, implied fractions such as molar mass (g/mol), percent by mass (g/100g), concentrations (mol/L or mol/kg) mole
fractions (mole/total mole) and even stoichiometric factors can be incorporated into a mathematical calculation in a single large fraction where each smaller fraction is divided from it’s neighbour by vertical lines in the larger fraction. Example, to convert from g of hydrogen to moles of hydrogen, we use the molar mass as a conversion factor. Note that we have written the molar mass conversion factor seemingly inverted here but that is what is required to cancel the units “g H” to leave the desired units “mol
H”. This method is first discussed in the course notes here.
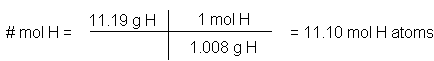
- First Law of Thermodynamics:
- Often called the Law of conservation of energy. There are several ways of stating this law:
- I. Energy can neither be created nor destroyed but only changed from one form to another.
or
II. The energy of the universe is constant.
or
III. The energy of a system which is isolated from its surroundings is constant. - Formal Charge
- The formal charge of an atom involved in bonding is a simple count of charge numbers associate with the atom, assuming that all the bonding is 100% covalent. Formal charge (FC) is defined mathematically as the core charge (CC) minus the number of valence electrons that can be associate with the atom.
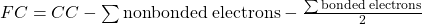
- Formation Reaction
- A reaction in which one mole of product is formed from its constituent elements in their standard state. This definition implies that there are no other products. For example, the formation reaction for methanol would have only one mole of methanol as the product and the elements carbon, hydrogen and oxygen as the only reactants, each in their standard state. C(s,graphite) + 2 H2(g) + 1/2 O2(g) → CH3OH(l)
- Hund’s Rule
- Hunds rule states that an atom is at its lowest energy state when it is at its highest total electron spin S for a given electronic configuration.
- ICE Table
- The ICE Table is a calculation technique used in various types of equilibrium problems. The columns of the table correspond to the species in the balanced chemical equation and the rows are as follows: (I) Initial activities, (C) Changes to initial necessary to reach equilibrium, and Final activities (F) = (I + C). The Final Activities are often algebraic expressions and can be substituted in to the equilibrium Constant expression as part of the solution.
- IF Table
- The IF table is a simplified ICE table. It is used in situations where we need to do a limiting reagent calculation, often in order to then use the results as the initial entries
for a subsequent ICE table. The rows of the IF table are: (I) initial activities and (F) final activities, where the final activities are calculated using the stoichiometry of the balanced chemical equation, as described in - Immiscible
- two liquids that do not form a solution when they are mixed are called immiscible. For example, oil and water are considered immiscible as they will not dissolve into each other. (see miscible).
- Insulated system
- An insulated system is designed so it does not lose heat to the surroundings or vice versa but it is not necessarily sealed against material transfer. For example, a Styrofoam coffee cup might lose material but will retain heat.
- Ionic Solids
- Ionic solids are made up of ions of opposite charge which hold together with electrostatic (Columbic) interactions.
- Isolated system
- An isolated system is both sealed against material transfer and insulated against heat transfer. For example, a thermos bottle is sealed so you don’t lose the contents and also insulated so the contents stay cold (or hot). Thus, No heat or matter can transfer between the system and its surroundings.
- Heat
- Heat is the Energy transferred between the system and the surroundings as a result of a temperature differential. This is the only kind of energy transfer that does not involve doing work. Heat is given the symbol q in equations. We often attach a subscript to the symbol to designate the special conditions under which the value applies. Thus, if the value applies to a constant pressure system, we use the symbol qp. A constant volume system undergoing a heat exchange would require the symbol qv.
- Heat Capacity
- Heat capacity of a substance describes the amount of heat that can be absorbed by the substance for a given unit rise in temperature (unit = 1 K). It can be expressed in general as:
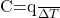 . Since heat, q, can be measured under different conditions (constant pressure or constant volume) we expect to find different values of the heat capacity of a substance held at constant pressure, CP, versus the heat capacity at constant volume, CV. Under these special conditions, we can define the heat capacity better using state functions as follows:
. Since heat, q, can be measured under different conditions (constant pressure or constant volume) we expect to find different values of the heat capacity of a substance held at constant pressure, CP, versus the heat capacity at constant volume, CV. Under these special conditions, we can define the heat capacity better using state functions as follows:
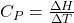 for constant pressure processes
for constant pressure processes
or
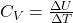 for constant volume processes.
for constant volume processes. - Kinetic Energy
- This term is used to describe the energy of motion. KE = 1/2 mv2
- Limiting Reagent
- The limiting reagent is the reagent in a chemical system that is used up first. Once one reagent is used up, the reaction cannot proceed further so the amount of product that can be produced is ‘limited’.
- Lewis Acid
- A Lewis acid is an electron pair acceptor in a chemical reaction with a Lewis Base. Click Here for a more detailed discussion.
- Lewis Base
- A Lewis base is an electron pair donator in a chemical reaction with a Lewis Acid. Click Here for a more detailed discussion.
- Lewis Dot Structures
- Lewis Dot structures are a way of modeling molecules. The atom cores are represented by the letter symbol for the element and the valence electrons are represented as dots or pairs of electrons, by short dashes. This model is a very rudimentary model of bonding in atoms and serves as a useful preliminary description of bonding in molecules.
- Metallic solids
- Metallic solids are made up of metal atoms, whose loosely held outer electrons, which are somewhat free of their positive cores and form a continuous dissociated sea of negative charge binding the positive cores together.
- Miscible
- Miscible is a word that describes the relationship between two or more liquids. Two liquids that dissolve into each other are called miscible liquids. The term can be modified to accommodate various scenarios. Fully miscible implies that the two liquids dissolve
into each other at any concentration. Partially miscible means the two liquids only dissolve into each other in a select range of concentrations (small amounts of A dissolves in B but if equal amounts of A and B are mixed we do not get a single solution, we see two phases). (See immiscible). - Molality
- A measure of concentration, useful in gravimetric measurements. Molality is defined as moles of solute divided by kilograms of solvent. Like molarity, this measure is only useful in dilute solutions where the distinction between solute and solvent is clear, based
on the relative concentrations of the two. The concentration of the solute is very small compared to the concentration of the solvent. - Molar Enthalpy Change
- The molar enthalpy change is used to measure the enthalpy released or absorbed in a chemical reaction. The term ‘molar’ can refer to a specific chemical in the balanced reaction or to the reaction itself. Thus, the molar enthalpy change ΔH of a chemical reaction has dimensions of Energy per mole, for which the SI units are kJ/mol.
- Molarity
- A measure of concentration most useful for volumetric measurement techniques. Molarity is defined as moles of solute divided by litres of solution. Like molality, this measure is only useful in dilute solutions where the distinction between solute and solvent is clear, based on the relative concentrations of the two. The concentration of the solute is very small compared to the concentration of the solvent.
- Molar heat capacity
- The heat capacity per mole is called the molar heat capacity (units J K-1mol-1)
- Mole
- The amount of a substance which contains as many elementary entities as there are atoms in exactly 0.012 kg (12g) of 12C
- Molecular Formula
- The chemical formula of a substance that forms molecules in which the exact number of each type of atoms is explicitly given. For example, the molecule benzene has a 1:1 ratio of C to H but has six of each atom in the molecule. the molecular formula for Benzene is C6H6. Many substances, such as ionic compounds and metals, do not form molecules and so cannot have a molecular formula. These substances can only be represented chemically using their emperical formula.
- Molecular solids
- Molecular solids consist of molecules that are held together by week intermolecular forces. A prime example of this is sulfur. Molecules of sulfur (S8) are held together by intermolecular forces far weaker than the covalent bonds that keep the atoms within each molecule.
- Molar mass
- The mass of one mole of a particular species. Generally, we refer to the molecular molar mass if the substance forms molecules, otherwise, we are referring to the emperical formula molar mass.
- Normal Hypervalence state
- Atoms with valence electrons in n>2 energy levels, also can have a normal valence state but can also have a hyper-valent state if there are unpaired electrons that can be split up to make more bonds. For example, consider the atoms in column 5 of the main-group blocks of the periodic table. They all have 5 electrons in the neutral atom. The normal valence state would be 3, i.e., 3 bonds, with one lone pair to add up to 5 valence electrons. Phosphorous, P, can also have a normal valence of 3 bonds with one lone pair. However, P has extra orbitals in the n=3 valence level (d-orbitals) and so it can make more bonds. If we split up the lone pair electrons, we can make a total of 5 bonds. Thus, the normal hypervalent state of atomic phosphorous, P, is 5 bonds with 0 lone pairs. It is always possible to imagine other valence states for P but any atom in a valence state other then the normal valence state and the normal hypervalence state will have a charge. For example, PCl4+ would have a P in a valence state of 4 bonds and no lone pairs. It would look like a carbon, with only 4 valence electrons (rather than the 5 that a neutral atom would have) so it would have a formal charge of +1.
- Normal Valence state
- The normal valence state for any atom is defined by the number of bonds and lone pairs that atom can form, using only the s or p orbitals of the valence energy level such that the formal charge of the atom is zero. Generally, atoms in a normal valence state follow the octet rule. For example, in the molecule CO2, double bonds connect the C to the two O atoms. The carbon is in its normal valence state of 4 bonds and no lone pairs and the oxygens each are in their normal valence state of two bonds and two lone pairs. This is in contrast to the hydroxide ion, OH–, where the oxygen has only one bond and three lone pairs. The oxygen’s valence state is a valid state, just not a ‘normal valence state’. In other words, the formal charge on the oxygen is not zero. Any atom whose valence electrons are in level n=2 has only one atomic valence state, called the ‘normal valence state‘. Atoms with valence levels with n>3, have d orbitals available and can create hypervalence states
- Normalization
- A normalization factor is simply a number that when multiplied onto the wave function, gives a probability of exactly 1 (for 1 electron).
- Octet Rule
- The octet rule states that atoms tend to bond together in a way that each atom can have associated with it exactly 8 electrons in 4 pairs. This ‘rule’ is useful in making Lewis Dot structures as a quick way to evaluate if the structure is correct. However, it is
superseded by considerations of formal charge and hypervalence states. - Open system
- An open system can transfer both material and heat, for example, a paper coffee cup loses both water (as vapour) and heat to the surroundings.
- Oxidation
- A chemical or element that loses an electron is said to be oxidized. It was first believed that oxidation involved the addition of oxygen to a substance, hence the name. Later, it was realized that oxidation involved the transfer of electrons and oxygen did not even need to be present.
- Potential energy
- This term describes the energy a system has that might be released as kinetic energy if a situation changes. For example, a book lifted against gravity to a high shelf distance h above the floor has more kinetic energy than it would on the floor. If the book falls off the shelf, that potential energy is converted to kinetic energy and then to sound and thermal energy as it strikes the floor. PE = mgh
- Rate
- The Rate of a reaction is defined to be simply the amount of chemicals reacted divided by the time it took to react. In reactions where the rate changes over time, and most do, this is only an average rate. Generally, we use concentrations in moles per Litre as the units we measure for ‘amount’. the Time can be in whatever units are convenient, seconds, days, years, … so we can define mathematically, the average rate of reaction to be
![Rendered by QuickLaTeX.com $Rate=\frac{\Delta[chemical]}{\Delta t}$](https://ecampusontario.pressbooks.pub/app/uploads/quicklatex/quicklatex.com-c2b01471b5d9043167a0a5c2219c2a32_l3.png)
- An instantaneous rate is better defined as
![Rendered by QuickLaTeX.com $Rate =\frac{d [chemical]}{d t}$](https://ecampusontario.pressbooks.pub/app/uploads/quicklatex/quicklatex.com-14352838d75adb19195617052db2932c_l3.png)
- In these two equations, the term [chemical] would be replaced by the concentration of a select chemical concentration with appropriate stoichiometric considerations multiplied in.
- Reaction Quotient
- The reaction quotient is calculated using the exact same formalism as is found in the thermodynamic equilibrium constant.
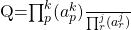 The difference between Q and K is that, the activities used to calculate Q are the activities of the system at the time of measurement, not necessarily
The difference between Q and K is that, the activities used to calculate Q are the activities of the system at the time of measurement, not necessarily
at equilibrium. In other words, Q can be calculated using component activities for a system that is not at equilibrium whereas K can only be calculated using the activities
of a system at equilibrium. - REDOX
- This is an acronym made up of the first letters of the words REDuction and OXidation. As implied by the conjunction of the two, reduction and oxidation can normally only occur simultaneously. It is only possible to separate oxidation from reduction using electricity and external circuits.
- Reduction
- A chemical or element that gains an electron is said to be reduced. The word ‘reduced’ seems strange but it refers to the early observations with regards to metal ores. When they are ‘reduced’ by smelting, i.e., they lose oxygens and so lose mass. So the word was a reference to the measurable reduction in the amount of material after it is reduced. Later, it was realized that oxygen need not be involved and simply transferring
electrons to a substance will ‘reduce’ it. - Repeatability
- The atom repeatability in a solid refers to how regular the arrangement of atoms is in that solid. Solids that have very regular, repeatable arrangements of atoms are crystals. The atom positions in crystals that have long-range repeatability can be determined from a unit-cell set of positions, repeated using some sort of symmetry operation such as translation along an axis by some number of unit cell widths along that
axis. Solids that have low repeatability are glasses and atom positions cannot be predicted using a basic unit cell set of atoms beyond one or two unit cell dimensions. - Second Law of Thermodynamics
- The second law of thermodynamics states that for a spontaneous process in a closed system that the entropy of the system must increase. In an open system, the entropy of the whole universe must increase.
- Specific heat
- The heat capacity per gram of a substance is called the specific heat capacity(units J K-1g-1)
- Standard Ambient Temperature and Pressure
- SATP is an unofficial standard and has T = 25℃ and P=100 kPa.
- Standard Enthalpy of formation
- The enthalpy change for a standard formation reaction
- Standard Temperature and Pressure
- STP is a standard used for gas condition calculations and is defined as T = 0℃ (273.15 K) and P = 100 kPa.
- Strong Acid
- A strong acid is one which dissociates 100% in solution.
Caveat:
This is a simplification of the fact that the amount of dissociation is actually dependent on the concentration of the acid in solution and on the solvent being used. Restricting ourselves to aqueous solutions and to concentration ranges that we typically use in experiments (100 to 10-6 M). If the acid is dilute enough it will fully dissociate as if it were a strong acid and if the acid is concentrated enough, it will not fully dissociate, even if it is
nominally a ‘strong’ acid. - Strong Electrolyte
- A strong electrolyte is one that ionizes fully in water solution. An electrolyte is a compound that dissociates into ions when dissolved in water. Acids and bases and salts are electrolytes. The term ‘strong’ refers to the extent of dissociation (ionization) in solution. See strong acid for further discussions of the concept.
- Surroundings
- After we have defined the system, the surroundings are defined by default. It is most simply defined to be the rest of the universe. Although, in practical experiments, we often refer to the surroundings as the rest of the room we’re in, or perhaps the rest of the chemical apparatus. For example, a large water bath surrounding a reaction chamber might be considered the surroundings if it is insulated from the rest of the universe.
- System
- The system is something we define ourselves and that definition then lets us write the equations we need and know what the symbols in the equations mean. The system is generally defined to be whatever we are interested in. That might be: the chemicals in a reaction, the reaction mixture, the chemicals and their container, etc. Essentially, we want to define the system in a way that makes the calculations as simple as possible.
- Thermodynamic Equilibrium Constant
- The equilibrium constant is used as a general descriptor of the conditions necessary for a system to achieve equilibrium. The most general form of the equilibrium constant is the Thermodynamic Equilibrium Constant, K . The value of K is determined using the relative activities of the products and reactants as follows.
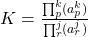 Where subscript p refers to the product components and subscript r refers to the reactant components and k and j are the coefficients of the reactants and products, respectively, in the balanced chemical equation and are, here, exponents of the activities of these same components. Consider the reaction jA + kB
Where subscript p refers to the product components and subscript r refers to the reactant components and k and j are the coefficients of the reactants and products, respectively, in the balanced chemical equation and are, here, exponents of the activities of these same components. Consider the reaction jA + kB 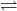 lC +mD The thermodynamic equilibrium constant for this equation is
lC +mD The thermodynamic equilibrium constant for this equation is 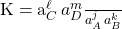 There are less general forms of the equilibrium constant that use units of concentration and/or pressure in their formalism. These are less useful forms and generally are only valid as comparative values, for example, to compare the equilibrium conditions of two different chemical systems. Only the thermodynamic equilibrium constant should be used in further thermodynamic calculations.
There are less general forms of the equilibrium constant that use units of concentration and/or pressure in their formalism. These are less useful forms and generally are only valid as comparative values, for example, to compare the equilibrium conditions of two different chemical systems. Only the thermodynamic equilibrium constant should be used in further thermodynamic calculations. - Thermodynamic Standard State
- The thermodynamic standard state (TSS) boils down to a simple condition; the activity of a substance that is in thermodynamic standard state equals unity (a = 1). More specifically, the TSS of a substance is the most stable form of that substance at P = 1 bar and concentration = 1 mol/L (for solutes) in solution. Temperature is not part of the definition although generally tabulated values of standard state parameters such as enthalpy, entropy, etc. are for a temperature of 25℃. TSS is indicated for such parameters using a superscript zero on the symbol for the thermodynamic parameter. For example, the Standard Enthalpy Change for a process is ΔH°, whereas the enthalpy change for a process not occurring at standard state is just ΔH.
- Thermodynamics
- The science of transformation of energy.
- Third Law of Thermodynamics
- The Third Law of Thermodynamics states that the entropy of a perfect crystal a absolute zero of temperature is zero. This serves to give us a zero point for our entropy measurements. Because of this law, we can know (theoretically, at least) the absolute entropy of any substance. This is in contrast with the concept of enthalpy, where we do not know the zero of enthalpy and hence we can never know the absolute enthalpy of any substance.
- Titration
- A titration is an analytical experiment whereby an amount of one chemical is measured using a known amount or concentration of a second chemical that will react with it. There are several types of titrations, some measure mass, others measure a volume of titrant. The Volumetric Titration is the most common type of titration technique encountered in first-year chemistry. Generally, the sample is placed in a flask (or beaker) and the titrant is added slowly from a measuring device, like a burette. The experiment is completed when the end point occurs. Volumetric titrations are done using different types of chemistry:
- An acid-base titration involves and acid titrated with a base. For example, the sample is an acid and the titrant is an base. The indicator is selected that will change color at the pH where all the initial acid is used up.
- A REDOX titration involves an oxidizing agent and a reducing agent. For example, the sample is the oxidizing agent and the titrant is the reducing agent. The indicator would be something that is added to the sample that will change color once the oxidizing agent is all used up (excess reducing agent).
Total electron spin
The total electron spin S is simply the sum of all the ms values for all the electrons in the atom. ![]() Generally, since most each filled orbital contains two electrons with spin ms = +1/2 and -1/2, respectively, the total electron spin is at or near zero.
Generally, since most each filled orbital contains two electrons with spin ms = +1/2 and -1/2, respectively, the total electron spin is at or near zero.
Atoms with an even number of electrons can obtain a total spin of zero if all orbitals are either exactly filled or completely unoccupied. Atoms with an odd number of electrons can not achieve a spin of zero. Thus, the ground-state beryllium atom (4 electrons) has a total spin of zero whereas the ground state boron atom (5 electrons) has a total spin of 1/2 If Hund’s rule kicks in then the atoms may have a total spin higher than zero, even if they have an even number of electrons. For example, Oxygen, with 8 electrons has a total spin of 1 because in its ground state, there are two p orbitals that have one electron each in them, with the spins parallel (both +1/2).
Valence
The valence of an atom (or ion) is simply a count of the number of bonds connecting that atom to another atom or atoms in a molecule or ion. For example, the molecule water, H2O, has one oxygen that is bonded to two hydrogens by single bonds. The valence of oxygen is therefore 2, while the valence each of the two hydrogens is 1. If the atom is to be neutral, then there is a limited number of possible valence states
. Valence Electrons: The valence electrons are the electrons in the outermost principle
energy level in the ground-state atom or ion involved in bonding. These electrons are generally distinguished from the core electrons, which are ‘not’ involved in bonding, at least in the simplest of bonding models.
.Weak Acid
A weak acid is one which forms equilibrium in solution, rather than dissociate 100%. Caveat: This is a simplification of the fact that the amount of dissociation is actually dependent on the concentration of the acid in solution and on the solvent being used. To use this definition, we should restrict ourselves to aqueous solutions and to concentration ranges that we typically use in experiments (100 to 10-6 M). If the acid is dilute enough it will fully dissociate as if it were a strong acid and if the acid is concentrated enough, it will act as a weak acid, i.e., not fully dissociate, even if it is nominally a ‘strong’ acid.
Weak Electrolyte
A weak electrolyte does not fully ionize when dissolved in water, it reaches equilibrium.
An electrolyte is a compound that dissociates into ions when dissolved in water. Acids and bases and salts are electrolytes. The term ‘weak’ refers to the extent of dissociation (ionization) in solution. See weak acid for further discussions of the concept.
Work
Work is one means of transferring energy from our system to the surroundings. Work is given the symbol w in equations. Work has several aspects to it. There is mechanical work, electrical work, light-energy work and work of expansion or contraction of a gas (PV work). In chemistry, we generally are only interested in PV work and will use the symbol w to refer only to that type of work. All other types of work will be referred to using a prime on the symbol, w’.

