17. Organic Reactions Problems
Organic Reactions Problems
1. Arrange the following in order from the worst to the best nucleophile.
- HCOO¯, HO¯, (CH3)2N¯
- CH3COO¯, H2N¯, HO¯
- (CH3)2N¯, HO¯ , CH3COO¯
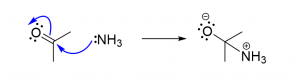
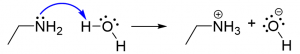
2. You are given two compounds, acetone and ammonia and asked to predict the products. Which of the following reaction schemes using curved arrows do you think gives the correct products? [C]
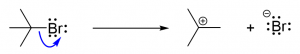
3. You are given the following compound and are asked to predict the product, based on the electron motion indicated by the curly arrows.
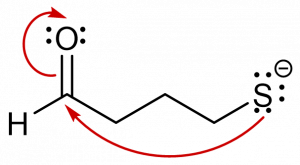
Which of the following represents the correct product? [C]
a. 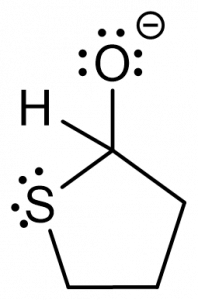 b.
b. 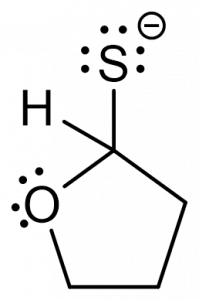 c.
c.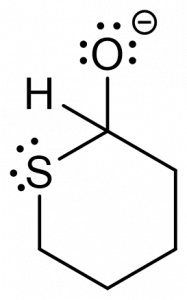 d.
d. 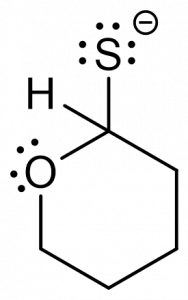
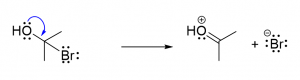
4. A reaction where one of the reactants is a chiral compound produces a single product that is the optical inverse of the original reactant. What kind of reaction is it? [b]
- SN1
- SN2
- Addition
- Dehydrogenation
- Lewis acid base reaction
5. A certain reaction produces two products that together form a racemic mixture. What kind of reaction is it? [a]
- SN1
- SN2
- Addition
- Dehydrogenation
- Lewis acid base reaction
6. What is the compound that will result when the following reactants are allowed to react? What kind of reaction is this?
(CH3)2C=CH2 + HCl → ?
- (CH3)2CH-CH2Cl
- (CH3)2CCl-CH3
- (CH2Cl)2C-CH3
- (CH3)2CCl-CH2Cl
- (CH3)3C-CH3
[b, Markovnikov addition]
6. Which of the following products is correct for the following reaction HClC=C(CH3)2 + HCl → ? What kind of reaction is this?
- CH3-C(CH3)2Cl
- CH3-C(CClH2)2
- CClH2-C(CH3)3
- CCl3-C(CH3)3
- CClH2-CH(CH3)2
[e, anti-Markovnikov addition]
7. Name the reaction type for the following reaction. HClC=C(CH3)2 + H2 → H2ClC=CH(CH3)2 .
- addition
- dehydrogenation
- hydration
- substitution
- hydrogenation
[a,e]
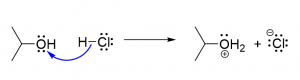
8. Which of the following reactions is an SN2 reaction?
- A reaction between a nucleophile and a chiral molecule produces a racemic mixture.
- A reaction between a nucleophile and a chiral molecule produces a single product with the symmetry inverted.
- A reaction between a substrate molecule and a base involves a carbocation intermediate and a single product.
- The reaction between a substrate molecule and a base occurs in a single concerted step involving both reactants.
- A nucleophile bonds to a carbocation to produce products.
9. Rank the following leaving group from strongest to weakest, a. Cl¯, b H3C¯, c H2N¯
10. Which of the following would make the best electrophile? [d]
- H2C=CH2
- (CH3)2S
- (CH3)3CH
- CH3CH2Br
11. Only one of the following reaction schemes is correct. Which is it? [a]
12. A hydrocarbon with a single double bond is heated in a cracking reaction. Draw all the products that might result. Note that there will be multiple products in some cases.