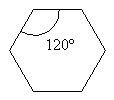2 Nomenclature
Michael Mombourquette
2.1: Introduction
This section will briefly outline the knowledge that will aid you in naming a few simple inorganic compounds. In order to understand chemical names, we will also need to understand several concepts of chemistry itself, including but not excluded to terms like: oxidation numbers, valence electrons and valence. These are briefly introduced here but are revisited in more detail later in the course.
The naming of chemical compounds is often known as nomenclature (accent on the syllable ‘men’). Before we can name a compound made up of multiple elements, we must learn to name the elements themselves. A list of commonly used elements is shown below. A complete list of chemical symbols may be found on the periodic table of the elements. Keep in mind that when the element symbol has two letters in it, they are always an upper case followed by a lower case letter.
e.g. The symbol for tin is Sn, not SN or sn or sN
When you write out the name of an element, you do not require a capital letter at the beginning of the name.
e.g. Sb is antimony not Antimony, unless you happen to be using the word to start a sentence.
Generally, the chemical name is taken from the name of the compound itself. Sometimes, the Latin name is used, often because when the particular element was first named, the English name was not in common use. In some cases, the first letter alone (carbon, oxygen, nitrogen, …) sometimes the first and second letters, (neon, nickel,…) sometimes the letter starting the first and the second syllables (lead [plumbum], silver [argentum], antimony [stilbium], neodymium,… ). As you can see, there is no simple rule so you will simply have to memorize the names that you will need.
Some commonly used elements are listed below. Watch the spelling. In a few cases, the Latin name for the element is used in the symbol lettering. For example, lead has the chemical symbol Pb from the Latin plumbum (think plumbing since originally, plumbing was done with lead pipes). Thus, the Latin name is given in parentheses in cases where the chemical symbol lettering comes from the Latin rather than the English name. Actually, Latin was the language of higher learning for many hundreds of years and so all early compounds were given Latin names. Most of our English names still use lettering that is close to consistent with the original Latin so most of the times, the letters are the same. In a few cases, like iron (ferrum), lead (plumbum), etc. our English names are not the same as the Latin names and hence the different letters. Other languages, such as French or Spanish, still use Latin names as their element names extensively and don’t have as big a problem.
| aluminum | Al | lead (plumbum) | Pb |
| antimony (stibium) | Sb | lithium | Li |
| argon | Ar | magnesium | Mg |
| arsenic | As | manganese | Mn |
| barium | Ba | mercury (hygrargyrum) | Hg |
| bismuth | Bi | neon | Ne |
| beryllium | Be | nickel | Ni |
| boron | B | nitrogen | N |
| bromine | Br | oxygen | O |
| calcium | Ca | phosphorus | P |
| carbon | C | potassium (kalium) | K |
| chlorine | Cl | radium | Ra |
| chromium | Cr | silver (argentum) | Ag |
| fluorine | F | sodium | Na |
| gold (aurum) | Au | sulfur | S |
| helium | He | tin (stannum) | Sn |
| hydrogen | H | uranium | U |
| iodine | I | zinc | Zn |
| iron (ferrum) | Fe |
Names and symbols shown in blue often cause difficulties.
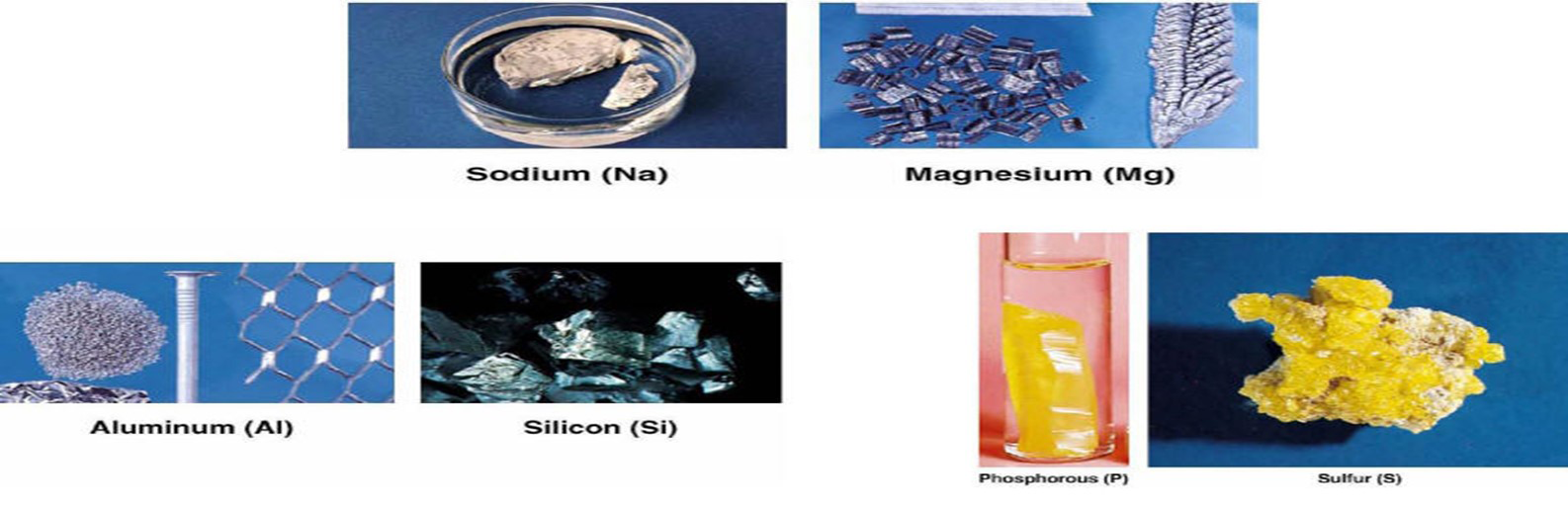
Some common elements are sodium, magnesium, aluminum, silicon, phosphorous, and sulfur:
2.2: Valence Shell Electrons
Valence shell electrons are those electrons found in the outer energy levels of an atom and are the only electrons capable of bonding. The bonding type depends on the extent to which bonding electrons are shared. In one extreme, electrons are not shared at all but are transferred wholly from one element (generally, the metal) to the a different atom (generally, the non-metal). This is called ionic bonding. The other extreme sees the electrons equally shared in a bond called a covalent bond. This latter type only occurs in homonuclear diatomic molecules. In all others, the bonding is somewhere in between the two extremes.
Bonding type may be determined by studying Lewis Structures and electronegativity values, as we’ll see later.
The most common number of valence shell electrons “involved in bonding” may be found using this guide. These numbers correspond to oxidation numbers shown on the periodic table or to the number of unpaired valence shell electrons in an element.
| Group | 1A | 2A | 3A | 4A | 5A | 6A | 7A | 8 |
|---|---|---|---|---|---|---|---|---|
| Common ionic charge | +1 | +2 | +3 | +4 | -3 |
-2 |
-1 |
0 |
The positive sign indicates that if an ion was formed during bonding, electrons would be lost by the element and the resulting ion formed would take on a positive charge.
The negative sign indicates that if an ion was formed during bonding, electrons would be gained by the element and the resulting ion formed would take on a negative charge.
The positive and negative charges would result only if ions are formed. Oxidation numbers give chemists a method of keeping track of electrons.
Generally, you can only determine an element’s oxidation number by comparing it with the other elements to which it is bonded. However, many elements have predominantly one particular oxidation number and simple rules can be used to determine its oxidation number. When you are unsure of an element’s oxidation number, one method is to look it up on the periodic table. Locate the column it is in and then you can determine its normal oxidation number.
Example: What are oxidation numbers of F, Ca, S, Fe?
The first three can be quickly determined from their column location in the periodic table.
F is in column VIIB and has OX# = -1
Ca is in column IIA and has OX# = +2
S is in column VIB and has OX# = -2 (like Oxygen). However, if S and O are bonded together then O will get the electrons (It attracts electrons more strongly than S, see the course section on electronegativity) and the S will end up being positive, not negative.
A more complete list of rules can be found in the e-text chapter 16.
This works well for main group elements but transition metals, like Fe are not so simple. There, oxidation numbers don’t follow quite as simple a set of rules. For example, Fe has two common oxidation numbers (+2; ferrous, and +3 ferric) but it also has others (+4 and +5 in the biologically important cytochrome P450) Similarly, the rules for counting electrons in transition metal complexes are not the same as for main group compounds. In this course, we will mostly be worried about main-group compounds with only a few transition group compounds that you can memorize.
2.3: Binary Compounds
A binary compound is a compound formed from two elements only. The name of any binary compound ends in “ide”.
If our binary compound is ionic (metal bonded to a non-metal), and if the metal has only one oxidation state, we can name them using simple rules.
The metallic element (found on lower left of the periodic table) is named first.
The actual oxidation number can be determined from the column in the periodic table. Column 1 had OX# = +1, Column 2 has OX# = +2. transition metals generally have more than one oxidation state and are not named using these simple rules.
The non-metallic element (found to the upper right of the periodic table) is then named, except that its ending is changed to “ide”. The ide ending means that element holds the negative oxidation number (negative charge in the pure ionic limit).
The oxidation number on the non metal can also be determined from the column in the periodic table. elements in column 7 will have OX# = –1, in column 6, OX# = –2, in column 5 it is –3 and anything else rarely forms ionic compounds.
Example:
- NaCl = sodium chloride
- KBr = potassium bromide
When writing formulae:
- For convenience, treat all compounds as ionic compounds, even though many are covalent.
- Compounds have no net charge. Therefore, positive and negative oxidation numbers (charges) have to balance one another.
Example: MgCl2 has one Mg2+ ion and two Cl1-ions, hence no net charge. - The positive ion in a compound is written before the negative ion.
Example: sodium chloride has the formula NaCl
Write the chemical formula for:
| a) potassium chloride | ________________________________ | Answers |
| b) sodium oxide | ________________________________ | |
| c) aluminum fluoride | ________________________________ | |
| d) copper oxide | ________________________________ | |
| e) iron chloride | ________________________________ |
Write the name of the following formulae:
| f) CaO | ________________________________ | Answers |
| g) BaI2 | ________________________________ | |
| h) Rb2O | ________________________________ | |
| i) Hg20 | ________________________________ | |
| j) HgO | ________________________________ |
Notice that d) and e) above have two formulae each. These two answers are easy to distinguish when looking at the formulae. However, we must be able to distinguish them by name.
Similarly, i) and j) have two different formulae yet they have the same name.
How do we eliminate these ambiguities?
2.4: -OUS -IC System
This system was used in compounds containing a metal with more than one oxidation number and a nonmetal. The simple binary naming system would not work because the different oxidation numbers gave different binary compounds with the same metal/non-metal pair. Metals in the first two columns of the periodic table have only one oxidation state and so will work find with the simpler rules discussed above. Other compounds cannot use the simple binary rules. For example; FeCl2 and FeCl3 are both binary compounds between iron and chlorine. Unfortunately, since iron had two different possible oxidation numbers, (+2) and (+3), respectively in these two compounds, we cannot name them both iron chloride since we need a unique name for each compound.
One early system for naming such multi-oxidation number metals uses suffixes like ‘ic’ and ‘ous’ to indicate oxidation state of the metal. Generally, this system was developed back in the days when Latin predominated as the language of science so the element names are the Latin ones. Hence, Fe2+ is ferrous and Fe3+ is ferric. This system was applied to several of the early-known transition metals and still is used to this date in a few common compounds. This system is largely superseded by other more versatile naming systems for the myriad of modern compounds that are known today.
Other elements that use the ‘ic’ ‘ous’ system include copper, lead, mercury, tin.
In this case, the ‘most common’ oxidation number must be memorized. For example, in iron, it is the 3+ oxidation state that is most common. Thus, any compound with iron (3+) must be named using the ‘ic’ suffix. So FeCl3 is ferric chloride. The next lower common oxidation form of the metal has the ending ‘ous’. Thus, FeCl2 is called ferrous chloride.
This naming method requires knowing Latin names of some elements. Here is a table of a few common metals and their oxidation numbers and names, which you may encounter in books and articles in your future studies. This course will not rely on or test your knowledge of these but you should be able to recognize them for easier understanding of your reading in the future.
| Element | Latin Name | Higher OX# | Name | Lower OX# | Name |
|---|---|---|---|---|---|
| copper | cuprum | +2 | cupric | +1 | cuprous |
| iron | ferrum | +3 | ferric | +2 | ferrous |
| lead | plumbum | +4 | plumbic | +2 | plumbous |
| tin | stannum | +4 | stannic | +2 | stannous |
We can see in this table a problem. The higher oxidation number in the ‘ic’ named element is not consistent and worse yet, the lower oxidation number is not always one lower. In lead, it is two lower than the higher one. The final blow to widespread use of this system involves the problem that many transition metals have more than two oxidation states (for example Vanadium has 4 different states as found in VO, V2O3, VO2, V2O5.)
This system largely relies on memorization of the names for each element. It will not be used in this course except in the most casual of ways and in any exam, you will always be given both the name and the formula so that you cannot lose marks for not having memorized this. That said, you will encounter it in your readings so let’s try a few examples.
| Cu2O is | _________________ | Answers |
| Plumbic sulfide is | _________________ | |
| FeCl2 is | _________________ | |
| Stannous nitride is | __________________ |
2.5: IUPAC System
A more generally used naming system called the IUPAC system (International Union of Pure and Applied Chemistry) was developed, which is completely general and does not require ambiguous memorized suffixes on element names.
If you are naming a chemical compound from a formula, and the first element in the formula has more than one oxidation number, the oxidation number for this multi-valent element is placed as a Roman Numeral, in parentheses, after the name of the element.
This method can be used to name any binary ionic compound but in the case of mono-valent metals, it is redundant and so not used.
| copper (I) oxide is | __________________ | answers |
| Hg3N is | __________________ | |
| FeCl2 is | __________________ | |
| iron (III) sulfide is | __________________ | |
| MgO | __________________ |
2.6: Prefix System
The third way of naming compounds is the prefix system. This method is almost always used when bonding occurs between two non-metals. To use this method you do not require oxidation numbers but must know the following prefixes.
| mono | 1 |
| di | 2 |
| tri | 3 |
| tetra | 4 |
| penta | 5 |
| hexa | 6 |
| hepta | 7 |
| octa | 8 |
| nona | 9 |
| deca | 10 |
When naming, place prefix indicating number of each type of atom in formula before the name of each element. Second element again has its ending changed to -‘ide’. If the prefix ‘mono’ appears in front of first nonmetal, it may be omitted. Thus, using this scheme, we would name CO2 as carbon dioxide and CO as carbon monoxide.
Name each of the following. Where applicable, give two names.
| 1. ZnS | _______________________________________________ | |
| Answers | 2. FeO | _______________________________________________ |
| 3. Sb2S3 | _______________________________________________ | |
| 4. CaCl2 | _______________________________________________ | |
| 5. BaO | _______________________________________________ | |
| 6. CuBr2 | _______________________________________________ | |
| 7. HgCl2 | _______________________________________________ | |
| 8. H2O | _______________________________________________ | |
| 9. PBr3 | _______________________________________________ |
Write the formulae for each of the following.
| 10. sodium chloride | _____________________________ | answers |
| 11. calcium bromide | _____________________________ | |
| 12. ferrous sulfide | _____________________________ | |
| 13. copper (II) iodide | _____________________________ | |
| 14 cuprous selenide | _____________________________ | |
| 15. manganese (II) oxide | _____________________________ | |
| 16. stannic sulfide | _____________________________ |
Some people have had fun with the names thusly derived. Look at the humorous DHMO web site, purporting to be a genuine research division.
2.7: Compounds With Polyatomic Ions
Not all compounds are binary. This can easily be noticed by flipping through a chemistry book and noting the names of many compounds not ending in ‘ide’. Compounds not binary generally contain a polyatomic ion consisting of a number of atoms. which hang around as a group. The group remains unchanged during a chemical reaction, and has a single oxidation number.
The key to remembering many of the polyatomic ions is knowing the five oxy-acids listed below. They will unlock thousands of chemical formulae for you.
OXY-ACIDS
| nitric acid | HNO3 | |
| carbonic acid | H2CO3 | |
| chloric acid | HClO3 | (iodic HIO3, bromic HBrO3) |
| sulphuric acid | H2SO4 | (selenic H2SeO4, telluric H2TeO4) |
| phosphoric acid | H3PO4 |
These five oxy-acids can be memorized using the memory tool “Nick the Camel ate Clams for Supper in Phoenix“, where the chemical symbol matches the underlined letters in the words. Note that other acids in the same family can be identified quickly. Thus for example, chlorine an be replaced by bromine or iodine (but not fluorine) to make other oxy-acids.
Additional other acids can be easily found for each entry here by adding and subtracting oxygen atoms to/from the above oxy-acids. A summary of these acids is shown below. Thus, we could have HNO, HNO2, HNO3. The key to naming these is in memorizing the ‘normal’ number of oxygens and using the ‘ic’ ending for that compound (chloric acid is HClO3). Any acid with one less oxygen uses the ‘ous’ ending (chlorous acid is HClO2). If two less oxygens are used the prefix ‘hypo’ (below) is used with the ‘ous’ ending (hypochlorous acid is HClO). If one extra oxygen is used the prefix ‘per’ (above) is used with the ‘ic’ ending (perchloric acid is HClO4)
Here are a few examples. Can you fill in the chemical formulae in the blank spaces in this table?
| Hypo_ous Acid | ous Acid | ic Acid | Per____ic Acid |
|---|---|---|---|
| HClO | HClO2 | HClO3 | HClO4 |
| ___ | HNO2 | HNO3 | ___ |
| ___ | H2SO3 | H2SO4 | ___ |
| ___ | H2CO2 | H2CO3 | ___ |
| ___ | H3PO3 | H3PO4 | ___ |
| <——– Subtract an O | Add an O ——–> | ||
| Answers | |||
| Examples of Acids: | HClO is hypochlorous acid |
|---|---|
| phosphorous acid is H3PO3 | |
| HClO4 is perchloric acid | |
| tellurous acid is H2TeO3 | |
| HIO2 is iodous acid | |
| selenic acid is H2SeO4 |
Polyatomic oxy-anions may be obtained from above acids by removing the H atoms from first part of formula. Oxidation number for polyatomic ion is equal in number to number of H’s found in the acid. A table of the polyatomic ions obtained from above acids is shown below.
Can you determine the correct formulae for these common polyatomic oxy-anions?
Table of Polyatomic oxy-anions Ions
| Hypo_ite | ite | ate | Per____ate |
ClO- |
ClO2- |
ClO3- |
ClO4- |
| ___ | NO2- |
NO3- |
___ |
| ___ | SO32- |
SO42- |
___ |
| ___ | CO22- |
CO32- |
___ |
| ___ | PO33- |
PO43- |
___ |
| <——– Subtract an O | Add an O ——–> | ||
| Answers | |||
When a polyatomic ions is part of chemical formula, the compound must still be electrically neutral. You must balance the charge supplied by polyatomic ion by adding the appropriate number of positive ions (metals).
e.g. the compound sodium phosphate needs 3 Na+ ions to balance PO43- to give us Na3PO4
Write a formula for each of the following:
- sodium sulphite
- magnesium carbonate
- aluminum hypochlorite
Give the name of each of the following:
- HNO2
- Ca3(PO3)2
In addition to the polyatomic ions obtained from the oxy-acids, there are a number of other ions commonly found in chemistry. You will encounter these ions from time to time and should know what they are.
| NAME | FORMULA OF ION | “OXIDATION NUMBER” |
|---|---|---|
| hydroxide | OH- |
1- |
| ammonium | NH41+ | 1+ |
| acetate | CH3COO-) |
1- |
| permanganate | MnO4- |
1- |
| chromate | CrO42- |
2- |
| dichromate | Cr2O72- |
2- |
| bicarbonate | HCO3- |
1- |
| bisulfate | HSO4- |
1- |
| cyanide | CN- |
1- |
| thiocyanate | SCN- |
1- |
| oxalate | C2O42- |
2- |
Write the formula for each of the following:
- potassium acetate
- ammonium oxalate
Write the name of each of the following:
- CuCr2O7
- Fe(SCN)3
2.8: Naming Organic Compounds
Organic chemistry involves the study of the chemistry of carbon-containing compounds (organic compounds). At first glance, one may think this to be a relatively small area of chemistry. In fact, Carbon-containing compounds comprise over 90% of all known compounds. This could be due in part to the fact that there are many areas of research in organic chemistry and thus, many compounds have been discovered. It is, however, largely due to the vast number of compounds that carbon and hydrogen (and a few other elements) form.
Organic compounds contain mostly hydrogen and carbon. Those compounds containing only these two elements are called hydrocarbons. Hydrocarbons can have single-, double-, and triple- CC bonds, they can form many branches and ring structures and form a large part of chemistry. More interesting in their reactivity, however are compounds that contain atoms other than these two. We call these other atoms heteroatoms to refer to the fact that they are other than hydrogen and carbon. Heteroatoms cause changes in the reactivity (or function) of the organic compound and the locations in the molecules where these groups exist are called functional groups. For example, a very common functional group that contains a single oxygen-hydrogen pair is the alcohol group. for example, methanol is CH3OH and ethanol is CH3CH2OH. Other functional groups include carboxylic acids -COOH, ethers Amines -NH3, etc. These actually make up the compounds of most interest to organic chemists.
2.9: Alkanes
The simplest class of organic compounds (but not limited to simple molecules) is called alkanes. An alkane is a compound made up of only hydrogen and carbon (hydrocarbon) that has only single bonds in it. Of these, the straight-chain hydrocarbons are the easiest to deal with. These compounds are called straight-chain not because the line of carbons forms a straight line but because they form a continuous line. The following table lists a few straight-chain hydrocarbons and gives names and properties
|
n |
Name |
BP |
MP |
Structural formula |
Formula |
|
1 |
methane |
-162 |
-193 |
CH4 |
CH4 |
|
2 |
ethane |
-89 |
-183 |
CH3CH3 |
C2H6 |
|
3 |
propane |
-42 |
-188 |
CH3(CH2)CH3 |
C3H8 |
|
4 |
butane |
0 |
-138 |
CH3(CH2)2CH3 |
C4H10 |
|
5 |
pentane |
36 |
-130 |
CH3(CH2)3CH3 |
C5H12 |
|
6 |
hexane |
69 |
-95 |
CH3(CH2)4CH3 |
C6H14 |
|
7 |
heptane |
98 |
-91 |
CH3(CH2)5CH3 |
C7H16 |
|
8 |
octane |
126 |
-57 |
CH3(CH2)6CH3 |
C8H18 |
|
9 |
nonane |
151 |
-54 |
CH3(CH2)7CH3 |
C9H20 |
|
10 |
decane |
174 |
-30 |
CH3(CH2)8CH3 |
C10H22 |
|
20 |
eicosane |
343 |
37 |
CH3(CH2)18CH3 |
C20H42 |
|
30 |
triacontane |
446 |
66 |
CH3(CH2)28CH3 |
C30H62 |
The general formula for alkanes is CnH2n+2. It is interesting to note that as the alkanes get larger, their boiling and melting points get higher. This makes sense on two counts: the heavier the molecule, a) the more energy it needs to be “lifted” out of the liquid phase, etc. and b) the larger chains get tangled in each other, making the un-tangling process nearly impossible.
- molecules of n = 1 – 5 are gas at room temperature (like methane, alias, natural gas)
- molecules of n = 6-16 are liquids at RT (like hexane, a major component of gasoline)
- if n >16 we see a waxy solid (like parowax)
2.10: Structural isomers
Alkanes can form many compounds, often, several compounds with the same chemical formula can have different structures. Compounds with this relationship are called structural isomers of each other.
For example, consider a hydrocarbon with four carbons, C4H10. This molecule can be represented as a straight-chain
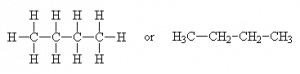
(common name: n-butane, IUPAC* name: butane)
or as a branched molecule
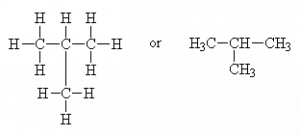 or CH3-CH-(CH3)2
or CH3-CH-(CH3)2
(common name: isobutane, IUPAC name: 2-methylpropane or just methylpropane; the 2 is redundant since there is no other place for a branch to go.)
These molecules both have the same chemical formula but are obviously different in their connectivity (structure). They are structural isomers of each other.
If we consider a five-carbon alkane, we can have three different isomers.
![]() ====> n-pentane
====> n-pentane
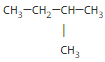 ====> iso-pentane alias 2-methylbutane or simply methylbutane
====> iso-pentane alias 2-methylbutane or simply methylbutane
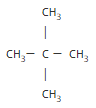 ====> neo-pentane or 2,2-dimethylpropane
====> neo-pentane or 2,2-dimethylpropane
These three molecules are all structural or compositional isomers of each other, i.e., they all have the formula C5H12 but they have different connectivities (structures).
Remember, these structural formulas are not real three-dimensional representations of the shape of the molecules themselves. They merely map out the way the atoms are connected. No information is implied regarding the orientation of these molecules based solely on structural drawings like these, except in special cases which we’ll get to later.
Thus, for example, the molecule methylbutane can equally well be represented as any one of the following structures (hydrogens not shown for simplicity).
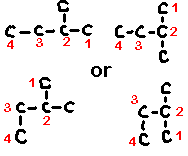
2.11: Conformers
In the previous discussion of structural isomers, we stated that the 3-D representation of the molecules was nearly completely neglected in the structural drawings we normally create to represent the various molecules. If we study for a moment the 3-D arrangement of atoms in a simple molecule, we see that this adds to the level of complexity that we need consider to completely understand a given molecule.
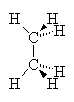
In the case of alkanes (no double bonds or ring structures) there is (in principle) free rotation around any of the single bonds in the structure (as around single bonds in any chemical structure). Thus, it is not correct to write down a single picture of the molecule and expect that that is how it always looks. If we consider the two-carbon alkane ethane we see that the Methyl groups can rotate around each other such that at one instant, the hydrogens of one methyl group are exactly aligned with those of the other group. This arrangement is called the eclipsed conformation since the hydrogens eclipse each other when viewed down the axis of the molecule. On the other hand, a slight rotation of one methyl group compared to another brings the hydrogens out of alignment to a conformation called staggered. In this case, all six hydrogens are visible when viewed down the molecular axis. Study the drawings and manipulate the 3D models below to satisfy yourself that you understand this.
| Eclipsed | Staggered |
|---|---|
 |
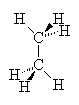 |
These two images show us instantaneous pictures of the geometry of this molecule, in reality, it is rotating so fast about the C-C bond that the two orientations swap more than 106 times per second. Some molecules with bulkier groups instead of hydrogens can find themselves trapped in one conformation or another. This can lead to observed differences in the properties of the two conformers (isomerism), as if they were in fact isomers.
Potential energy of different conformations:
In order for molecules to reach their lowest possible potential energy, the individual atoms must all be free to find the best positions that optimizes the overlap of orbitals. if there are near-neighbor atoms that interfere with this freedom of motion, there can be strain on the bonds such that the molecule has a less than optimal potential energy (higher PE). In the two molecule conformations above, the eclipsed conformation would be higher in PE than the staggered because the steric interaction of the two hydrogens is greater when they are eclipsed than when they are staggered, i.e., they bump into each other more. If there were other groups like methyl or ethyl groups then we would see even more steric interaction and hence, even higher PE as these groups interact more.
In these later cases, we call these different conformations “conformational isomers”, since there would be a whole series of differing potential energies, depending on eclipsed versus staggered and also depending on which groups are next to each other.
2.12: Cycloalkanes
A further step in the complications of structures of alkanes involve the possibility that a ring structure may be formed. If no C=C double bonds exist these compounds are called cycloalkanes. They have the general formula (CH2)n. Let us consider just the first few cycloalkanes.
In these conformations, the carbon atoms can assume more closely the ideal tetrahedral geometry of the sp3– hybridized atoms they are.
2:13: Naming (Nomenclature)
It is obvious that using the chemical formula alone is not sufficient to uniquely identify the vast majority of organic compounds. Structural formulas too are difficult to use and impractical to put in spoken language. Originally, names for compounds were not well coordinated and there were often compounds that had been named by different people completely different things. It was very hard to determine exactly what chemical the names were referring to without first researching the history of each individual name. A structured naming regime called IUPAC is now in place to help us both name new compounds and rebuild the structure of named compounds. This set of rules helps us to uniquely identify each chemical compound by its name alone. One drawback to this scheme is that the names can become quite long. For a more complete listing of the IUPAC rules than I can give in these lecture notes, click here,
Let’s look at some of the rules to help you learn how to use this nomenclature scheme.
1. Parent Chain
Select the longest continuous ‘chain’. It is the parent chain. It’s name is used as the last part of the compounds name. If there are several “longest” chains within the molecule of the same length then the parent chain will have the most possible branches and will have the lowest possible branch numbers of all the choices. Take, for example, the molecule pictured below (C6H14).
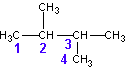 |
The longest straight chain is a four carbon chain (Numbered in blue). There are several possible choices for the four-carbon chain. It makes no difference which you pick. I chose the four carbons (blue numbered). Avoid the erroneous thinking that the ‘chain’ must be linear along the paper. This is not the case. Check out the 3D model and prove this for yourself.
Hence, the last part of the name for this example is butane
2) Numbers
Number the carbons in the parent chain (and in the branches) such that the branches (and any other non-alkane features like double bonds, hetero-atoms, etc.) occur at the lowest possible number carbon. Start with the first branch, if there are two ways to number the parent such that the first branch occurs on the same number then chose the one which gives the smallest numbered second branch, etc. In the above example, the numbering sequence could have been reversed with no difference in the location of the branches.
3) Branches
The branch names are those of the normal alkane of the same length but with the -ane suffix replaced by -yl (indicating a molecular fragment) thus, methane becomes methyl for a one-carbon chain, etc.
You now prefix the parent name with the chain names, indicating their location on the parent chain. In the above example, there are two methyl chains, located at carbons 2 and 3 on the parent chain. Hence, we use the prefix 2,3-dimethyl to describe the location and type of branches on the parent.
In this example, the completed name is 2,3-dimethylbutane. NOTE: no spaces in the name.
4) Double bonds and triple bonds
Double bonds and triple bonds are indicated by changing the ane to ene and yne, respectively, in the name of the chain. They should be included as part of the parent chain, even if a longer chain is possible but which excludes this functional group. The location of the functional group (double or triple bond) is indicated as the first carbon in the chain that has that type of bond.
Thus, the following 4-carbon compounds are named accordingly
| CH2=CH-CH2-CH2 | 1-butene |
| CH3-CH=CH-CH3 | 2-butene (Note that there are cis and trans isomers of 2-butene. See Geometric Isomers below) |
5) Cycloalkanes
Cyclic alkanes should be the parent chain in a compound (unless there is a “straight-chain” part that is larger or which contains an important functional group). If more than one cycle, then the largest one is the parent. Prefix the normal alkane name with “cyclo-” to make the name. Thus, a three-carbon ring is cyclopropane (see above) and a four-carbon ring is cyclobutane (see above). If there are substituents or branches on the ring, then numbering of the carbons is done such that they occur at the lowest possible numbered carbons. See the drawing below as example.
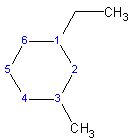
1-ethyl-3-methylcyclohexane
(Note: always put the substituents in alphabetical order and name it so. Also note that there is an alternate naming scheme where the largest substituents is first. I prefer the former method)
In the case where the acyclic part is longer than the cyclo part then the cyclo part is named as a cycloalkyl branch.
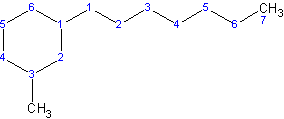
In this case, the cycloalkyl is called 3-methylcyclohexyl and it’s on carbon 1 of a heptane parent chain. Thus the name is 1-3-methylcyclohexylheptane.
6) Arenes
Arenes are a special class of organic compounds. They have what is called a conjugated p-bond system that rapidly resonates, creating a large delocalized orbital. This large delocalization creates an especially stable molecule towards certain types of reactions. The most common arene is a six-member ring of carbons where each carbon has one hydrogen on it, called benzene.
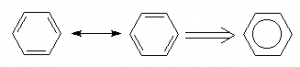
The alternating double and single bonds seen in the two resonance structures to the left of the double arrow ![]() helps us to see the number of bonds but don’t properly indicate that all carbon-carbon bonds are in fact equal in all respects. The overall resonance can be represented by the right-hand diagram where the double/single bonds are replaced by the circle indicating that the ring is aromatic. This, however is not a “Lewis Structure” in the classical sense since you cannot tell how many bonds each carbon has.
helps us to see the number of bonds but don’t properly indicate that all carbon-carbon bonds are in fact equal in all respects. The overall resonance can be represented by the right-hand diagram where the double/single bonds are replaced by the circle indicating that the ring is aromatic. This, however is not a “Lewis Structure” in the classical sense since you cannot tell how many bonds each carbon has.
Substituent groups can exist on these rings, numbering is such that they occur at the lowest possible numbers. Let’s look at some specific examples:
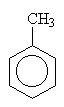 |
methylbenzene (1-methylbenzene is redundant) (Common name toluene) |
 |
 |
 |
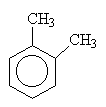
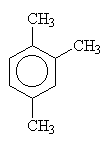
| 1,2-dimethylbenzene (orthodimethylbenzene) |
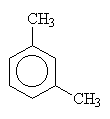
1,3-dimethylbenzene (metadimethylbenzene) |
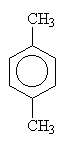
1,4-dimethylbenzene (paradimethylbenzene) |
Note that there is an alternate form of naming the location of the substituents on a benzene ring. This is not strictly IUPAC but is very commonly used. The prefix ortho indicates the adjacent position, meta indicates two positions away and para indicates directly across from (3 positions away).
 |
 |
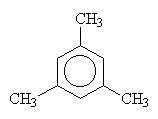 |
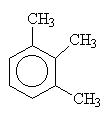
| 1,2,3-trimethylbenzene | 1,2,4-trimethylbenzene | 1,3,5-trimethylbenzene |
There are also molecules with multiple aromatic rings. Look a these few.
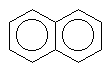 |
naphthalene |
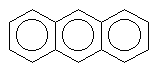 |
anthracene |
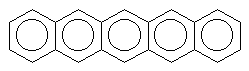 |
pentacene (blue) |
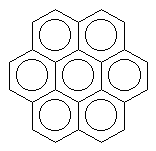 |
coronene (yellow) |
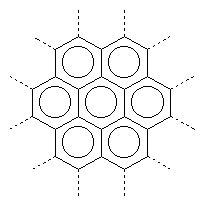 |
graphite (black) |
Here are a few examples, (Answers)
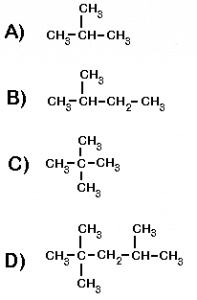
E )
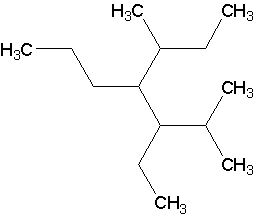
Now, draw the structures corresponding to the following names (Answers).
a) hexane
b) 2-methylpentane
c) 3-methylpentane
d) 2,2-dimethylbutane
d) 2,3-dimethylbutane
Note that these are all structural isomers of each other.
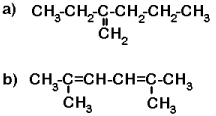
Now try a few with multiple bonds. (Answers).
2.13.1: Branched Alkyls.
As we have seen above, it is possible to get branches on the branches of our alkanes. These branched alkyls must also be named using the same rules we’ve seen above for the main-chain alkanes.
- Identify the longest part of the branch and use it as the root of the branch.
- Number the carbons on the branch (this time you must always start from the carbon attached to the main chain.
- Identify and number each branch off this branch using rules 1 and 2 recursively.
- name the entire branch using the identified alkyl components and then attach the whole branch using the rules above.
Take for example, the two formulas below.
There are a few common names that are often used as a part of a “IUPAC” name. If you recall the molecule isobutane (see above) which consisted of a three-carbon chain with a methyl group on the 2 position. It is more correctly named 2-methylpropane or just methylpropane (the 2- is redundant). There are many molecules that contain a group that could be classified as a three-carbon chain attached at position 2 rather than position 1. Take for example, the following two molecules.
Molecule 1 has a three carbon branch joined at the end to carbon 4 of the main chain. Since a three carbon chain has the root “prop” and this chain is a branch (suffix of yl) it is a propyl group. although it serves no purpose this time, the carbons on this group are numbered as shown, starting with the propyl carbon that’s bonded to the main chain.
| 1 | 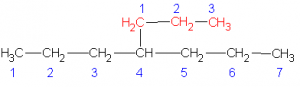 |
4-propylheptane |
| 2 | 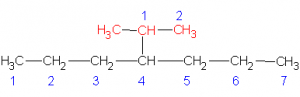 |
IUPAC: 4-1-methylethylheptane
or use the shortcut 4-isopropylheptane |
We can also look at the name of the three-carbon group in molecule 2. In this case, the propyl group is not attached at the end. It’s attached in the middle. We must start numbering from the carbon attached to the main chain, thus, we find a two-carbon chain with a methyl branch on carbon 1. Hence the IUPAC name is 4-1-methylethylheptane. In this case, we can use the shortcut prefix “iso” to give this branch a simpler name. Rather than a 1-methylethyl group, we can call it an isopropyl group and thus the name of the molecule is shortened to 4-isopropylheptane.
If we have a 4-carbon alkyl group, rather than the three carbon one above, there are more possibilities.
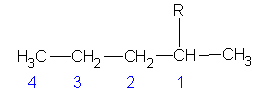
Recall that there are 2 structural isomers of the 4-carbon alkane. These two can each attach to a larger main chain in two ways. They can use a terminal carbon or a middle carbon. Thus we can find 4 different 4-carbon alkyl groups are possible. In the following molecules, I will not draw the main chain. I will represent it merely as R (the Rest of the molecule)
| #C | Alkane | Alkyl | Name |
|---|---|---|---|
| 4 | butane |
butyl | |
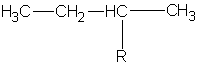 |
1-methylpropyl or sec-butyl s-butyl* |
||
| isobutane (2-methylpropane) 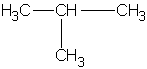 |
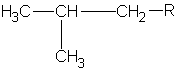 |
2-methylpropyl or isobutyl |
|
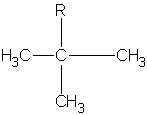 |
1,1-dimethylethyl or tert-butyl t-butyl tbutyl* |
||
| 5 | pentane |
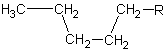 |
pentyl |
 |
1-methylbutyl | ||
2-methylbutane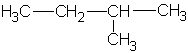 |
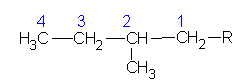 |
2-methylbutyl | |
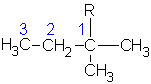 |
1,1-dimethylpropyl | ||
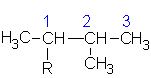 |
1,2-dimethylpropyl | ||
| 2,2-dimethylpropane or neopentane 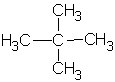 |
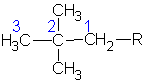 |
2,2-dimethylpropyl or neopentyl |
* In a couple of cases, we have some prefixes we’ve never seen before. Prefixes sec- and tert- (or sometimes t-) refer to secondary and tertiary, respectively. These terms refer specifically to the number of carbons in the branch attached directly to the root (carbon 1)
A carbon is considered a primary carbon if it has only 1 other carbon attached to it on the branch In this case, the carbon is at the end of a chain. For example the ethyl group ![]() can only have a primary carbon (in red). The propyl group could have a primary
can only have a primary carbon (in red). The propyl group could have a primary ![]() or a secondary
or a secondary 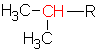 carbon, depending on where the R is attached. In the case of the secondary carbon (in the branch called 1-methylethyl), there are 2 other carbons bonded to that carbon. (we don’t use the name sec-butyl for this alkyl group since a more common shortcut is isobutyl as is indicated above.
carbon, depending on where the R is attached. In the case of the secondary carbon (in the branch called 1-methylethyl), there are 2 other carbons bonded to that carbon. (we don’t use the name sec-butyl for this alkyl group since a more common shortcut is isobutyl as is indicated above.
The butyl group can have a primary, ![]() , a secondary,
, a secondary, 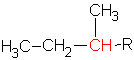 , or a tertiary carbon,
, or a tertiary carbon, 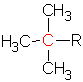 , depending on the structure. The tertiary carbon is one with three other carbons attached.
, depending on the structure. The tertiary carbon is one with three other carbons attached.
2.13.2 Geometric isomers.
There are times when molecules can have the same formula and the same structure (same connectivity) but are not the same molecules. The difference lies in how the substituents occupy the space in the molecule. These are classed into a general category called Stereo Isomers. Of these, Geometric Isomers forms a sub group.
Let’s try an example. What is the name of this molecule?
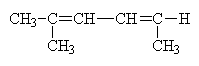
Actually, this example is more complex than it seems. The molecule, at first glance seems simpler than example b a few pages back. This is not the case. Because there is no free rotation about the double (or triple) bonds it is possible to have different isomers where the structure of the isomers is identical but where the geometric arrangement is not the same. Thus, we create geometric isomers. This is different from the conformers that we saw earlier where free rotation easily (some times) converts one geometric arrangement into another. Here, the geometric arrangements are not changeable without breaking a bond.
Thus, example b) above should actually be better drawn as follows:
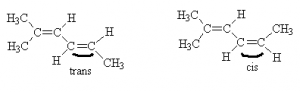
We can see two distinct isomers because the position of the CH3 group relative to the ethylene group on the other side of the (right=hand) double bond is different from one case to another. In one case, the non-hydrogen constituents are on opposite sides of the double bond. This is the trans configuration. In the other case, the non-hydrogen constituents are on the same side of the double bond. This is the cis configuration. thus, the names for these two molecules are trans-2-methyl-2,4-hexadiene and cis-2-methyl-2,4-hexadiene, respectively. Of course, there are always more complex cases were these simple rules break down and need refinement. We can’t cover all the rules in just a few hours worth of lectures.
Note that although there were two double bonds in these isomers, only one of them (highlighted) contributed to cis/trans isomerization. The other double bond in each group (not highlighted) have identical groups on one end (methyls) and thus, there can be no cis/trans isomers formed about that bond.
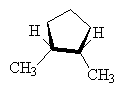
It is also possible to get cis-trans isomers from other molecular structures where rotation about a bond is not possible, for example, in cyclo- compounds, the ring prevents free rotation as follows.
|
|
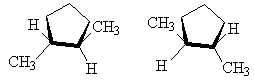 |
| cis-1,2-dimethylcyclopentane | trans-1,2-dimethylcyclopentane (1S,2S ) and (1R,2R ) |
NOTE: The two drawings on the right are both the same structure and geometry and therefore with the same name. However, they are not quite identical. They are in fact optical isomers (enantiomers) of each other. Chemically, they are identical (except to enantiomeric reactants and catalysts like enzymes) but optically they are different. Optical isomers can only form if there is 3-d structure in the mode (for example tetrahedral carbons). If the molecule has planar symmetry (trigonal planar carbons) then any mirror image is also super imposable. A simpler set of enantiomers can be found in the VSEPR chapter where I discussed a tetrahedral carbon with 4 different substituents on it.
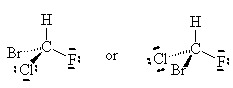
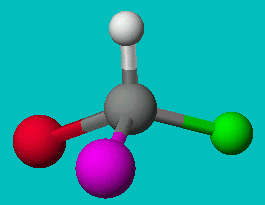 or
or 
Can you superimpose these mirror images?
video: non-super imposable mirror images
video: superimposable mirror images
These molecules are optically active (they rotate plane-polarized light). The pair are enantiomers (optical isomers) of each other. Individually, any molecule that displays optical activity is called chiral and the amount and direction of it’s optical activity is termed it’s chirality.
2.14: Functional groups
Functional groups (other than double and triple bonds) occur whenever a heteroatom (not carbon or hydrogen) is incorporated into the structure. The following is a quick list of functional groups and some quick information on naming them.
2:14.1: Alcohols
Alcohols are identified by presence of an OH group on a hydrocarbon chain (R–OH). These compounds are usually named by appending -ol to the end of the name and sometimes, indicating the location of the functional group using numbers as previously described. Below are a few examples of alcohols and their names.
| ethanol (note: remove the ‘e’ of ‘ane’) | |
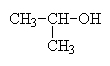 |
2-propanol (the OH is on carbon 2 of the propane chain) also called isopropanol. |
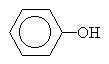 |
benzenol also called phenol |
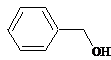 |
benzyl is a benzene with a methyl group. This is benzyl alcohol. |
There are several types of alcohols, ethanol and methanol have their functional group on a terminal carbon and are called primary alcohols (the carbon to which the OH is attached has at most one other carbon attached to it. R-COH.
Secondary alcohols occur when the functional group occurs in the middle of the chain, as in 2-butanol. Here, the alcoholic carbon has two other carbons bonded to it, hence the terminology secondary.
2-propanol could also be named accordingly as sec-propanol. (sec means secondary).
Tertiary alcohols involve alcoholic carbons with three other carbons bonded to them. In this case, the OH occurs just at the junction between two alkane chains as in:
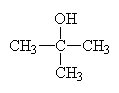 |
2-methyl-2-propanolor t-butanol (t is for tertiary) |
There are also diols and triols (and more), where more than one OH group exists on a single carbon chain.
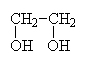 |
1,2-ethanediolor ethylene glycol (antifreeze) |
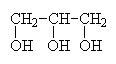 |
1,2,3-propanetriolor glycerol or glycerine |
2.14.2: Ethers
An ether group contains two hydrocarbon chains connect by an oxygen atom (R–O–R’). Simple ethers can be names by simply naming the two branches first and then prepending them to the word ether. Alternatively, more complex molecules can be named by considering the R–O group to be the alkoxy group and simply treating it like a branch on the larger molecule. Below are a few examples, some use both naming conventions. Numbering if necessary is done in the normal fashion.
| diethylether | |
| ethylmethylether or methoxyethane | |
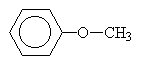 |
methylphenylether or methoxybenzene |
2.14.3: Aldehydes
An aldehyd has a terminal C with a double bonded oxygen (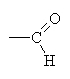 ).
).
such compounds are named by adding the suffix –al. Numbering is not necessary since the functional group must be (by definition) at the end of the carbon chain, therefore, it is always attached to carbon #1.
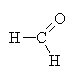 |
methanal |
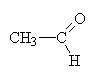 |
ethanal |
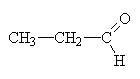 |
propanal |
Notice that these molecules are all drawn with the aldehyde carbon looking to have bond angles of about 120°. That’s because the C=O unit means the carbon is SP2 hybridized and is therefore trigonal planer with bond angles of approximately 120°. It’s best to represent the true geometry of at least the planar portions of molecules when you can and we do here.
2.14:4 Ketones
Ketones occur when a non-terminal carbon contains a double bonded oxygen (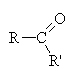 ).
).
Here, the R chains may or may not be identical, hence the prime on one of them. Here, however, we treat the entire chain (R-C-R’) as a single unit when naming and simply indicate the carbon number where the double bonded oxygen occurs (if necessary).
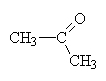 |
propanone (the 2 is redundant since 1-propanone is, in fact propanal.) This is a structural isomer of propanalcommonly called acetone (nail-polish remover). |
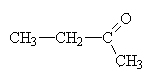 |
butanone. (again, the 2 would be redundant since 3-butanone is merely 2-butanone improperly numbered and 1-butanone is really butanal). |
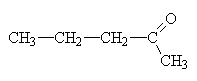 |
2-pentanone. There is also a structural isomer of this called 3-pentanone so the 2 is necessary in this name. |
2.14:5 Carboxylic acid
In this functional group, we combine the OH and the C=O on one carbon and the new functional group is a carboxylic acid(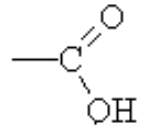 ).
).
This group is obviously always located on the terminal carbon of a hydrocarbon chain. It is named using the name of the hydrocarbon parent chain and the suffix -oic acid. Below, find some examples, alternative common names are give in parenthases.
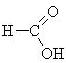 |
methanoic acid (formic acid) |
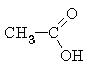 |
ethanoic acid (acetic acid) |
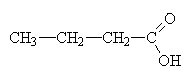 |
butanoic acid (butric acid) |
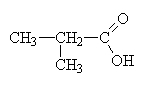 |
2-methylpropanoic acid (isobutric acid)This is a structural isomer of butanoic acid. |
2.14.6: Esters
An ester is made when a carboxylic acid and an alcohol react as follows
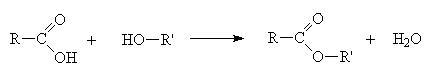
When naming an ester, we use the names of the component alcohol and of the carboxylic acid and append the suffix –oate.
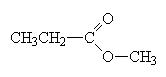 |
methylpropanoate |
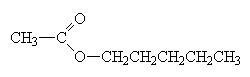 |
pentylethanoate (banana smell) |
2.14.7: Amines
Amines are compounds containing nitrogen, cf., ammonia (NH3). There are three types of amines.
Primary amines have only 1 carbon attached to the nitrogen. Secondary amines have two carbons attached to the nitrogen and tertiary amines have three carbons attached to the one nitrogen atom.
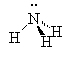 |
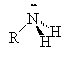 |
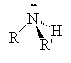 |
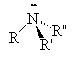 |
| ammonia | primary amine | secondary amine | tertiary amine |
We name the amines by simply naming each of the R groups alphabetically as we do branched on an alkane and ending the name in amine.
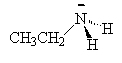 |
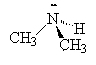 |
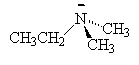 |
| ethylamine | dimethylamine | ethyldimethylamine |
In some cases, the groups attached to the nitrogen are more important (or complex) that the amine and naming is simplified by treating the amine as the branch rather than the parent. In this case, we name the amine and append the letter o to the end and then name the parent.
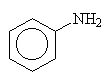 aminobenzene (could also be phenylamine but this is not customary).
aminobenzene (could also be phenylamine but this is not customary).
Other amines simply use the common name since other conventions become too complicated.
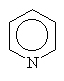 |
pyridine |
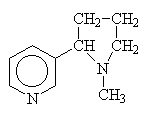 |
nicotine |
2.14.8: Amides
These are the compounds that hold your DNA and RNA in their double helix shape. An amide is like a carboxylic acid but where the OH group is replaced by an amine group, for example, NH2 or NHR or NRR’.
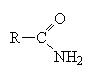
These molecules are easier to name. Simply name the carbon chain and then add the suffix amide.
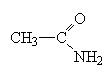 |
ethanamide also called acetamide (like acetic acid) |
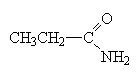 |
propanamide |
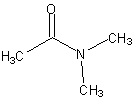 |
N,N-dimethylethanamide also called N,N-dimethylacetamideNote: we specify that the alkyl groups are on the Nitrogen using N. |
Additional Resources:
Note: this section and the accompanying work sheets are courtesy of Bill Newstead.
Answers to in-Chapter questions:
________________________________________________________
Binary Compounds
- KCl
- Na2O
- AlF3
- CuO or Cu2O
- FeCl3 or FeCl2
- calcium oxide
- barium iodide
- rubidium oxide
- mercury oxide
- mercury oxide
-OUS -IC System
Cu2O is cuprous oxide
Plumbic sulfide is PbS2
FeCl2 is ferrous chloride
Stannous nitride is Sn3N2
copper (I) oxide is Cu2O
Hg3N is mercury (I) nitride
FeCl2 is iron (II) chloride
iron (III) sulfide is Fe2S3.
MgO is magnesium oxide. magnesium(II) oxide would be redundant. You don’t need to use magnesium(II) to indicate that magnesium has a +2 oxidation number as that is the only oxidation number possible so you can figure out the oxidation number on Mg from the formula, knowing that the OX# on oxygen is –2.
| 1. ZnS | zinc sulfide |
| 2. FeO | iron monoxide, iron(II) oxide, ferrous oxide |
| 3. Sb2S3 | diantimony trisulfide or antimony (III) sulfide |
| 4. CaCl2 | calcium chloride |
| 5. BaO | Barium oxide |
| 6. CuBr2 | copper dibromide, copper(II) bromide, cupric bromide |
| 7. HgCl2 | mercury dichloride, mercury (II) chloride, mercuric chloride |
| 8. H2O | dihydrogen oxide or water |
| 9. PBr3 | phosphorous tribromide, phosphorous (III) bromide |
In cases where there is only one name given with no prefixes or roman numerals, it means that the metal has only one oxidation state and simply naming the elements is sufficient to create a unique name.
| 10 sodium chloride | NaCl |
| 11 calcium bromide | CaBr2 |
| 12 ferrous sulfide | FeS |
| 13 copper (II) iodide | CuI2 |
| 14 cuprous selenide | Cu2Se |
| 15 manganese (II) oxide | MnO |
| 16 stannic sulfide | SnS2 |
| Hypo__ous Acid | __ous Acid | ic Acid | Per____ic Acid |
|---|---|---|---|
| HClO | HClO2 | HClO3 | HClO4 |
| HNO | HNO2 | HNO3 | HNO4 |
| H2SO2 | H2SO3 | H2SO4 | H2SO5 |
| H2CO | H2CO2 | H2CO3 | H2CO4 |
| H3PO2 | H3PO3 | H3PO4 | H3PO5 |
| <——– Subtract an O | Add an O ——–> | ||
Write a formula for each of the following:
- sodium sulphite (aka sodium sulfite) is Na2SO3 because we need two Na+ for every one sulfite (SO32- ) ion.
- magnesium carbonate is MgCO3 because we need one Mg2+ for every CO32- ion.
- aluminum hypochlorite is Al(ClO)3 because for every one Al3+ ion, we need three hypochlorite (ClO–) ions
Give the name of each of the following:
- HNO2 is nitrous acid.
- Ca3(PO3)2 is calcium phosphite.
| Hypo__ite | __ite | __ate | Per____ate |
|---|---|---|---|
ClO-1 |
ClO2-1 |
ClO3-1 |
ClO4-1 |
NO-1 |
NO2-1 |
NO3-1 |
NO4-1 |
SO2-2 |
SO3-2 |
SO4-2 |
SO5-2 |
CO-2 |
CO2-2 |
CO3-2 |
CO4-2 |
PO2-3 |
PO3-3 |
PO4-3 |
PO4-3 |
<——– Subtract an O |
Add an O ——–> |
||
Write the formula for each of the following:
- potassium acetate ==> KCH3COO
- ammonium oxalate ==> (NH4)2 C2O4
Write the name of each of the following:
- CuCr2O7 ==> copper (II) dichromate or cupric dichromate
- Fe(SCN)3 ==> iron (III) thiocyanide or ferric thiocyanide
- 2-methyl propane
- 2-metnylbutane
- 2,2-dimethylpropane
- 2,2,4-trimetnylpentane
- 3-ethyl-2,5-dimethyl-4-propylheptane. There are numerous ways to get a 7-carbon chain. The parent is the one with the most branches directly on it.
b) 2-methylpentane 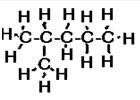
c) 3-methylpentane 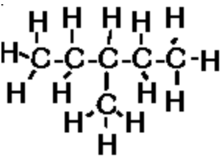
d) 2,2-dimethylbutane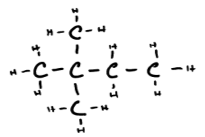
d) 2,3-dimethylbutane 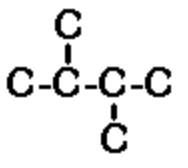
b) 2,5-dimethylhexa-2,4-diene