19 4.6. Family and Genera Abundance in Alzheimer’s Disease
Learning Objectives
- Understand the relation of microbial richness and diversity to the progression of Alzheimer’s disease
- Understand the role of the gut-brain-axis
- Gain insight into the mechanism of action of lipopolysaccharides (LPS) and understand its possible role in Alzheimer’s
- Understand which Genera & Families seem to be more abundant in Alzheimer’s disease
- Gain insight on how such abundance is similar to other medical conditions (eg. type-2 diabetes & Parkinson’s disease)
Bacterial Abundance in the Gut Microbiome and Alzheimer’s Disease
Based on scientific research, the gut microbiome of Alzheimer’s patients has a decreased amount of microbial richness and diversity in comparison to age-and-sex matched control groups. A decrease in microbial abundance is identical to previous observations made in other conditions that are associated with gut microbiome alterations; these include obesity, diabetes, inflammatory bowel disease (IBD), and Parkinson’s disease. A healthy gut microbiome is dependent on high microbial richness and diversity, as the immune system is positively correlated with a more capable bacterial community. To put this into perspective with a real-world example, think of a healthy microbiome as a successful company that has enough workers (all with distinct capabilities) to complete all required tasks successfully. Now, whilst the type of bacteria responsible for microbiome alterations changes from one condition to the other, it is believed that these alterations, which scientists call “dysbiosis”, are key to disease development; as these changes activate the immune system and lead to systemic inflammation.
The Association Between Bacterial Genera and Alzheimer’s Pathology
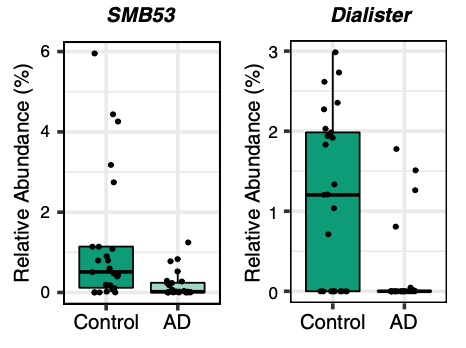
A correlation exists between the levels of abundance in the gut microbiota and CSF biomarkers, which explains their relation to Alzheimer’s pathology. The genera that are more abundant in Alzheimer’s are associated with greater Alzheimer’s pathology, whilst, genera that are less abundant in the disease are linked to less disease pathology. To measure Alzheimer’s pathology, the CSF must be examined for the ratio of p-tau/Aβ42, which are the two main hallmarks of the disease. Dialister (genus of Firmicutes bacteria) and SMB53 (genus belonging to the family of Clostridiaceae) demonstrate the greatest association in healthy individuals. A greater abundance of these two bacterial genera is linked to less disease pathology, which may indicate that they have protective properties against the progression of Alzheimer’s.
The Gut-Brain Axis and Broad Taxonomic Differences Between Alzheimer’s Disease and Control Groups
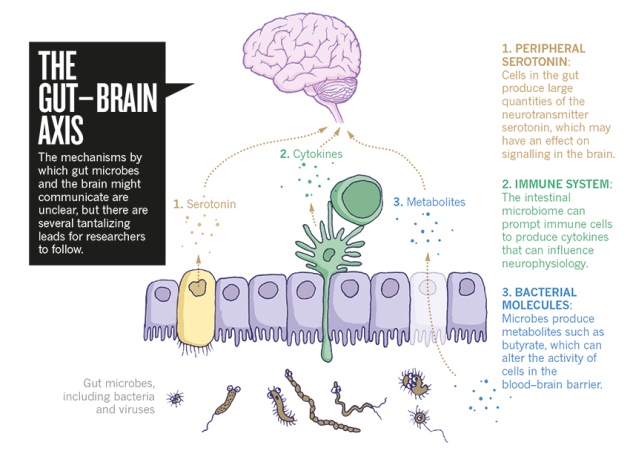
As aforementioned, the different levels of abundance in genera is associated with CSF biomarkers of Alzheimer’s pathology. Using bioinformatics, it was demonstrated that there are functional changes in metabolism, bacterial cell motility, and cell communication in the gut microbiome of Alzheimer’s disease patients, in comparison to healthy individuals. The exact way the gut affects the progression of neuropathology is still unclear. However, scientific data reinforces the idea of a gut-brain-axis, which allows for communication between the gut and the brain. The pathways that allow for this bi-directional communication include neural, endocrine, and immune systems. Thus, changes in microbial communities within the gut of patients with Alzheimer’s may lead to pathophysiological changes in the brain. In recent animal studies, transgenic mice models of Alzheimer’s disease that were raised in germ-free conditions possessed less amyloid deposition in the brain, than the same mice models raised in standard conditions. From this data, it may be concluded that the gut microbiome influences amyloid pathology.
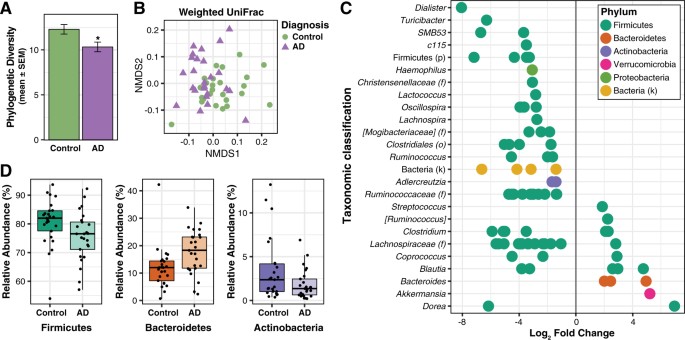
There are distinct taxonomic differences between Alzheimer’s patients and healthy individuals that are linked to CSF biomarkers of Alzheimer’s disease. Within the phylum Firmicutes, the family Gemellaceae, and the genera Blautia, Phascolarctobacterium, and Gemella are present in greater abundance in Alzheimer’s patients. Whilst, the families Ruminococcaceae, Turicibactereaceae, Peptostreptococcaceae, Clostridiaceae, and Mogibacteriaceae, and the genera SMB53, Dialister, Clostridium, Turicibacter, and cc115 all seem to be less abundant in Alzheimer’s disease. Similarly, a decrease in Firmicutes has been associated with the gut microbiota of individuals with type-2 diabetes and obesity; which along with insulin resistance increases the risk of developing Alzheimer’s disease. Insulin resistance is directly linked to a decrease in cerebral glucose metabolism and increased amyloid deposition. Therefore, insulin resistance is a possible source of gut microbial alterations influencing Alzheimer’s pathology.
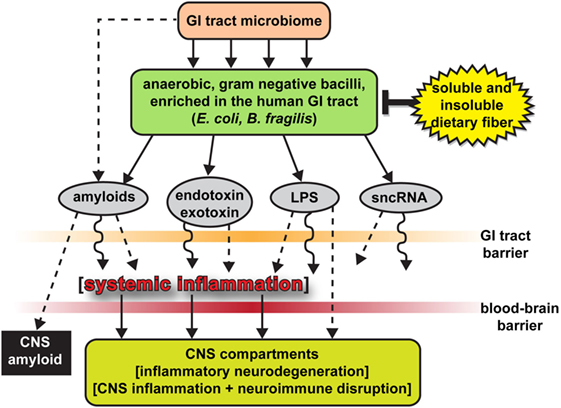
In Alzheimer’s patients, there is also an increase in the bacterial phylum Bacteroidetes, which is composed of a diverse selection of gram-negative commensal bacteria, including the genus Bacteroides and Alistipes, and at the family level Bacteroidaceae and Rikenellaceae; which has also been examined at high levels in the microbiome of patients with type-2 diabetes and Parkinson’s disease. Thus, it is important to investigate the role of lipopolysaccharide (LPS), which is a key part of gram-negative bacteria. LPS is able to trigger systemic inflammation and release pro-inflammatory cytokines after being translocated from the gastrointestinal tract to the systemic circulation.
The Significance of Lipopolysaccharide and Actinobacteria
There have been in vitro and in vivo studies conducted to show the link between bacterial endotoxins (such as LPS) and Alzheimer’s disease pathology. The co-incubation of amyloid-beta peptides with LPS generated the potential for amyloid clumping; and the injection of LPS in both wild-type and transgenic mice models of Alzheimer’s disease resulted in an increase of greater amyloid deposition and tau protein pathology. In relation to the gut microbiota, the intestinal permeability of an individual increases with age, and the elderly demonstrate a connection between greater LPS-binding protein (which acts as a marker of microbial translocation) and systemic inflammation. To add to the accumulation of evidence, studies have shown that the brain tissue collected from patients with Alzheimer’s shows co-localization of LPS and amyloid plaques. Thus, the increase in abundance of gram-negative bacteria like Bacteroides in patients with Alzheimer’s may lead to the translocation of LPS (found in the gut) to the systemic circulation, contributing to disease pathology. In comparison to healthy individuals, patients with Alzheimer’s exhibit a decrease in Actinobacteria. The changes in abundance come from alterations in Bifidobacterium, which are key bacterial inhabitants of the gastrointestinal tract, due to their beneficial properties, including anti-inflammation, decreased intestinal permeability, decreased LPS levels, and improved gut mucosal barrier.
The Importance of the Gut Microbiome in Alzheimer’s Disease
Understanding the association of the gut microbiome with the progression of Alzheimer’s disease may lead to new approaches in the field that may have the potential to decrease the risk or symptoms of this disease. The key is understanding the alternations within the gastrointestinal tract, and determining how restorations to a healthy bacterial community could be used as a protection against Alzheimer’s. Ultimately, there are three key ways by which the gut microbiota may affect the nervous system and influence the progression of neurodegenerative diseases. This includes immunomodulation, changes in an individual’s metabolism, and the direct influence on nerve cells and their signaling. Based on the aforementioned data regarding the relative abundance of bacterial Families and Genera, it is important to recognize the certain imbalances present in Alzheimer’s disease, and how changes in bacterial communities may play either a detrimental or protective role.
