11 2.4 Future directions: Neutrophil Involvement in ARDS
Learning Objectives
2.4.1 Neutrophils
2.4.1.1 General Characteristics
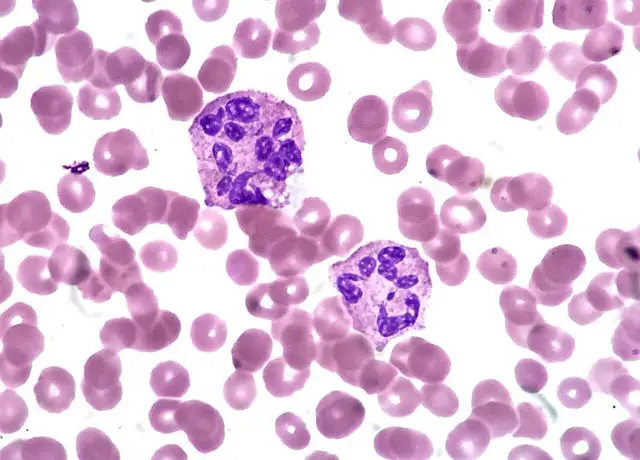
Neutrophils – also known as neutrophilic granulocytes or polymorphonuclear neutrophils (PMNs) – are the most abundant white blood cell (WBC) involved in the body’s immune response (Rosales et al. 1). They make up about 55-70% of your white blood cells. They are characterized by a multi-lobed nucleus (hence where the name ‘polymorphonuclear’ stems from), with 3-5 lobes joined together by genetic material (Eberl 1). They are also the smallest in size in the class of cells called granulocytes, which release granules when fighting pathogens (Kobayashi et al., 510).
Killing Mechanisms
PMNs are most prominently involved in the innate immune response (the arm of immunity that fights pathogens before an active infection). They are one of the first cell types at the site of infection, and are often recruited within seconds of injury (Rosales et al. 1). Neutrophils employ various mechanisms in order to fight pathogens. Neutrophils often recognize PAMPs (pathogen-associated molecular patterns) on the surface of the pathogen using their PRRs (pattern-recognition receptors; Rosales 1). They can also use Fc receptors, opsonization, and complement receptors to detect and identify pathogens (Kobayashi 508; Rosales 1). Using this receptor-mediated process, the pathogen is then internalized into a phagosome – or a specialized vacuole (Kobayashi 508). Neutrophils then release granules within the cytoplasm containing enzymes to help kill and digest foreign microbes (Kobayashi 508-510).
Another method neutrophils employ to kill pathogens is through a mechanism of neutrophil extracellular traps, or NETs (Rosales 1; Selders et al. 54). This involves the neutrophil releasing its sticky DNA (sticky after the sugar molecules on the DNA interacting with water in the environment) and trapping the pathogen (Selders et al 54). Other immune cells such as macrophages may then be recruited to continue the immune response (Selders et al 56).
2.4.1.2 Neutrophil Recruitment
Chemokines
Chemokines are a family of small cytokines (ranging from 8-12 kDa) that function to attract white blood cells, such as neutrophils, to the site of infection through a process called chemotaxis (“Chemokines: Introduction”). They are involved in a variety of other processes, such as regulating lymphoid organ development and T-cell differentiation (“Chemokines: Introduction”). Chemokines can be further divided into four families based on their arrangement of amino acid terminal cysteine residues: CXC, CC, C, CX3C; “Chemokines: Introduction”). CXC and CC chemokines form the largest group and will be the main focus in our discussion of ARDS. Of particular interest, CXCL8 is one of the most potent chemoattractant molecules responsible for guiding neutrophils to the tissue matrix (Williams et al. 66).
Chemotaxis
Chemotaxis is the process in which immune cells are recruited to sites of acute inflammation via chemokine gradients (Petri & Sanz 425). In order for a cell to respond to a chemokine and participate in chemotaxis, they must express a complementary chemokine receptor (“Chemokines: Introduction). Chemokine receptors tend to belong to the family of G-protein coupled receptors (GPCRs), which are characterized by seven transmembrane receptors that bind extracellular ligands (such as chemokines) and initiate intracellular signaling (“Chemokines: Introduction;” Petri & Sanz 430). This then initiates a signalling cascade that results in the activates of GTPases (“Chemokines: Introduction;” Petri & Sanz 430). Downstream effects include the activation of integrins (molecules that are involved in cell adhesion) and a series of intracellular processes that ultimately initiate cell movement (“Chemokines: Introduction;” Petri & Sanz 430).
Neutrophil Recruitment via Chemotaxis
CXCL8 is the chemokine particularly involved in neutrophil recruitment (specifically in ARDS) and is recognized by CXCR1 and CXCR2 expressed on the surface of neutrophils (William et al. 69). Once these CXCL8 interacts with these receptors, they activate a G-protein that in turn activates a second-messenger system (Petri & Sanz 427). Neutrophils, which are usually circulating in the blood (William et al. 69), are then ‘signalled’ to leave and are guided to the tissue matrix of interest, where they are then able to kill pathogens and remove cellular debris (Petri & Sanz 427). The process in which they migrate from the blood to tissues will not be covered here.

Key Takeaways
- Neutrophils are predominantly involved in the innate immune response and use a variety of methods to fight pathogens, including the release of granulocytes or NETs.
- They attracted to sites of infections by chemokines through a process of chemotaxis. CXCL8 is highly established chemokine involved with neutrophil recruitment, especially in acute respiratory distress syndrome (ARDS).
Concept Check
2.4.2 The Role of Neutrophils in ARDS
All information below has been acquired from the research study conducted by Williams and colleagues (2016).
2.4.2.1 Neutrophil Influx
Neutrophil influx into the extravascular compartments of the lungs is characteristic of ARDS (Williams et al, 66). Circulating neutrophils that become primed by chemokines are recruited to airspaces, where they then employ killing mechanisms.
The number of neutrophils correlates with disease severity and mortality rates (Zemans & Matthay 1). A significant increase in neutrophils can cause an increase in lung epithelial and endothelial permeability as a result of toxic mediators (their granules) that cause tissue injury (Zemans & Matthay 1). This leads to an influx of protein-rich alveolar oedema and arterial hypoxemia (Zemans & Matthay 1). This situation represents a double-edged sword; although the recruitment of neutrophils is important and necessary, too much can be toxic to individuals.
2.4.2.2 The Importance of CXCL8
Research investigating the neutrophil recruitment in ARDS pathogenesis have observed an important role played by one of the most potent chemoattractants, CXCL8. CXC class chemokines are in general found to be elevated in ARDS patients, however, CXCL8 are most predictive of disease development, severity, and mortality (Zemans & Matthay 1). CXCL8 is responsible for most (but not all) of neutrophil recruitment in ARDS (William et al. 2016).
Potential CXCL8 Synergism with CCL2 and CCL7
Research conducted by Williams et al. (2016) investigated the role of CXCL8, CCL2, and CCL7 interactions in neutrophil recruitment in ARDS patients. It is well established that CXCL8 plays an important role in ARDS pathogenesis, however, other chemokines have been overlooked. In order to develop therapies for ARDS, it is important to understand the complete and complex nature of neutrophil recruitment in this disease. The main results of this study is outlined below.
CCL2 and CCL7 are elevated in BAL fluid
The researchers first found that aside from CXCL8, CCL2 and CCL7 were elevated in bronchoalveolar lavage fluid (BAL fluid or BALF) in individuals challenged with LPS (an endotoxin of gram-negative bacteria) compared to saline challenged volunteers. In addition, concentrations of these cytokines were found to be elevated in patients with ARDS compared to both the LPS-challenged and saline-challenged individuals. This suggested that these two cytokines were likely playing a role in ARDS pathogenesis (but what?)
CCL2 and CCL7 significantly contribute to chemotactic potency in ARDS BAL fluids
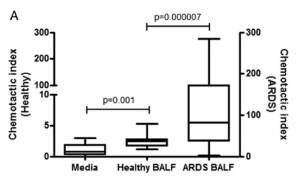
Once researchers found that CCL2 and CCL7 were elevated, they further investigated the potential role they were playing (i.e. why were these cytokines elevated? What were they doing?). What they found was these two cytokines had chemotactic potencies for neutrophils. Patients with ARDS were found to have highly chemotactic cytokines for neutrophils in their BALF compared to healthy volunteers. This conclusion was derived from the researchers neutralizing either CCL2 or CCL7 using antibodies. In both cases this resulted in a decrease of neutrophil chemotaxis towards ARDS BALF (Figure 3a).
Of particular interest however, was the result of neutralizing either a combination of CXCL8 and CCL2 or CXCL8 and CCL7. Researchers found that after doing this in the BALF fluid of ARDS patients, there was a cumulative decrease in neutrophil chemotactic response (Figure 3b-c). This then led to the hypothesis that CCL2 and CCL7 were working in synergy with CXCL8 in recruiting neutrophils to the airspaces of ARDS patients.
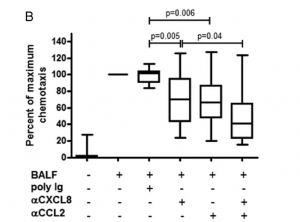
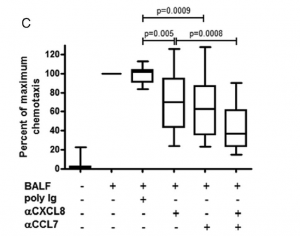
CCL2 and CCL7 enhance neutrophil chemotaxis to CXCL8
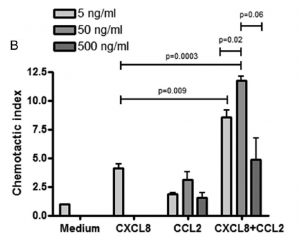
To provide further evidence of the synergy hypothesis, the researchers looked at the pattern of neutrophil recruitment with respect to either CXCL8, CCL2, or CCL7 alone. CXCL8 was found to recruit neutrophils in a concentration-dependent manner, whereas both CCL2 and CCL7 alone did not cause significant chemotaxis over a range of concentrations.
However, when either CCL2 or CCL7 was combined with CXCL8, there was a significant increase in neutrophil recruitment compared to alone. This further suggested CCL2 and CCL7 were working in synergy with CXCL8 to recruit neutrophils.


Neutrophils from ARDS BAL fluid modify their chemokine receptor expression
Recall that neutrophils express both CXCR1 and CXCR2 on the surface of their cells to interact with CXCL8 in their environment. An interesting finding of the William et al. (2019) study was that these receptors are plastic and change when moving from blood to airspaces in ARDS. To summarize briefly, it was observed that CXCL8 receptors on blood neutrophils were expressed highly, but once in BALF (i.e. airspaces of the lung) there was a significant decrease in CXCR1.
Key Takeaways
- Neutrophil influx into the extravascular compartments of the lungs is characteristic of ARDS and is associated with disease severity and mortality rates.
- CXCL8, an established chemokine involved in neutrophil recruitment, is thought to work in synergy with CCL2 and CCL7 to recruit neutrophils in ARDS pathogenesis.
Concept Check
2.4.3 Implications for future therapies
2.4.3.1 The importance of heterogeneity
Ideally, therapies against ARDS targeting neutrophils would limit their recruitment, priming, and activation, as the increase in such can result in serious tissue damage. However, the host defense would also need to remain preserved (Zemans & Matthay 2). In order to do so, it is important to understand the role of each mediator in this complex mechanism. ARDS is a heterogeneous disease and thus, therapies will need to be personalized to the individual patient. In the Williams et al. (2016) study outlined above, there was substantial variability in chemokine levels, the extent of each chemokine’s overall chemotactic activity of the BALF, and the neutrophil expression of CXC and CC chemokine receptors. Future therapies will need to be personalised to inhibit one or more mediators of neutrophil function seen in the patient while permitting the activity of others to allow the nature host defense (Zemans & Matthay 2).
2.4.3.2 What Remains Unknown
Future studies should aim to investigate some of the following questions:
- To what extent are CCL2 and CCL7 directly chemotactic? The authors of this study saw is was small, but not statistically significant. To what exact extent it contributes to the chemotaxis of neutrophils in synergy with CXCL8 in left unknown.
- Are there other chemokines that work in synergy with CXCL8, CCL2, and CCL7? CXCL5 and CXCL15 are also common neutrophil chemoattractants; do they contribute at all to ARDS?
- DO CCL2 and CCL7 work in other pathways in ARDS, such as activating alveolar macrophages or epithelial cells to secrete CXC chemokines?
- To what extent are these processes able to clear bacteria and other foregin debris in the alveolar spaces?
Conclusion
Neutrophils and their interactions with chemokines play an important and complex role in acute respiratory distress syndrome (ARDS). While CXCL8 has previously been an established and potent chemokine in the recruitment of neutrophils, other cytokines such as CCL2 and CCL7 may have been overlooked. Recent studies have demonstrated that CCL2 and CCL7 may be working in synergy with CXCL8 to recruit neutrophils to airspaces in ARDS. This is very important to consider when developing new therapies to treat ARDS; as a heterogenous disease, therapies will be required to be personalized to the individual in order to be effective.
Modern Day Implication: COVID-19-Induced ARDS
SARS-CoV-2 is the virus responsible for causing the current and ongoing pandemic. It has tropism for ciliated airway cells and type-II pneumocytes (Acosta & Singer 7). Using its S-protein (also known as the spike protein), this virus binds to the ACE2 receptor on host cells to mediate viral fusion and endocytosis (Acosta & Singer 6). It is then able to replicate within host cells by synthesizing its viral proteins (Acosta & Singer 6).
The mortality of SARS-CoV-2 infection is often induced via the development of viral pneumonia-induced ARDS, which develops in 42% of patients presenting with COVID-19 pneumonia and 61-81% o those requiring intensive care (Gibson et al. 1). COVID-19’s host immune response tends to consist of elevated serum cytokines such as IL-1beta, IL-6, and TNF-alpha, as well as impaired interferon responses, and elevated C-reactive protein, ferritin, and coagulation factors (Nemchand et al. 2). Characteristic of the infection is the induction of a cytokine storm, which often leads to the progression of ARDS (Nemchand et al. 2). Those present with COVID-19 ARDS have worse outcomes than those with ARDS from other causes (Gibson et al. 1).
In a case study presented by Nemchand and colleagues (2020), a 50 year old man presented with COVID-19 induced ARDS as a result of cytokine storm effects (and a state of hyperinflammation) and multi-organ failure. In an attempt to treat this man, they administered Anakinra, a recombinant form of a IL-1R antagonist. The goal of this treatment is to inhibit the activity of IL-1. This treatment had positive effects in treating the man, who survived the infection.
