63 6.5 Future Directions for NSCLC
Moving Towards Precision Oncology
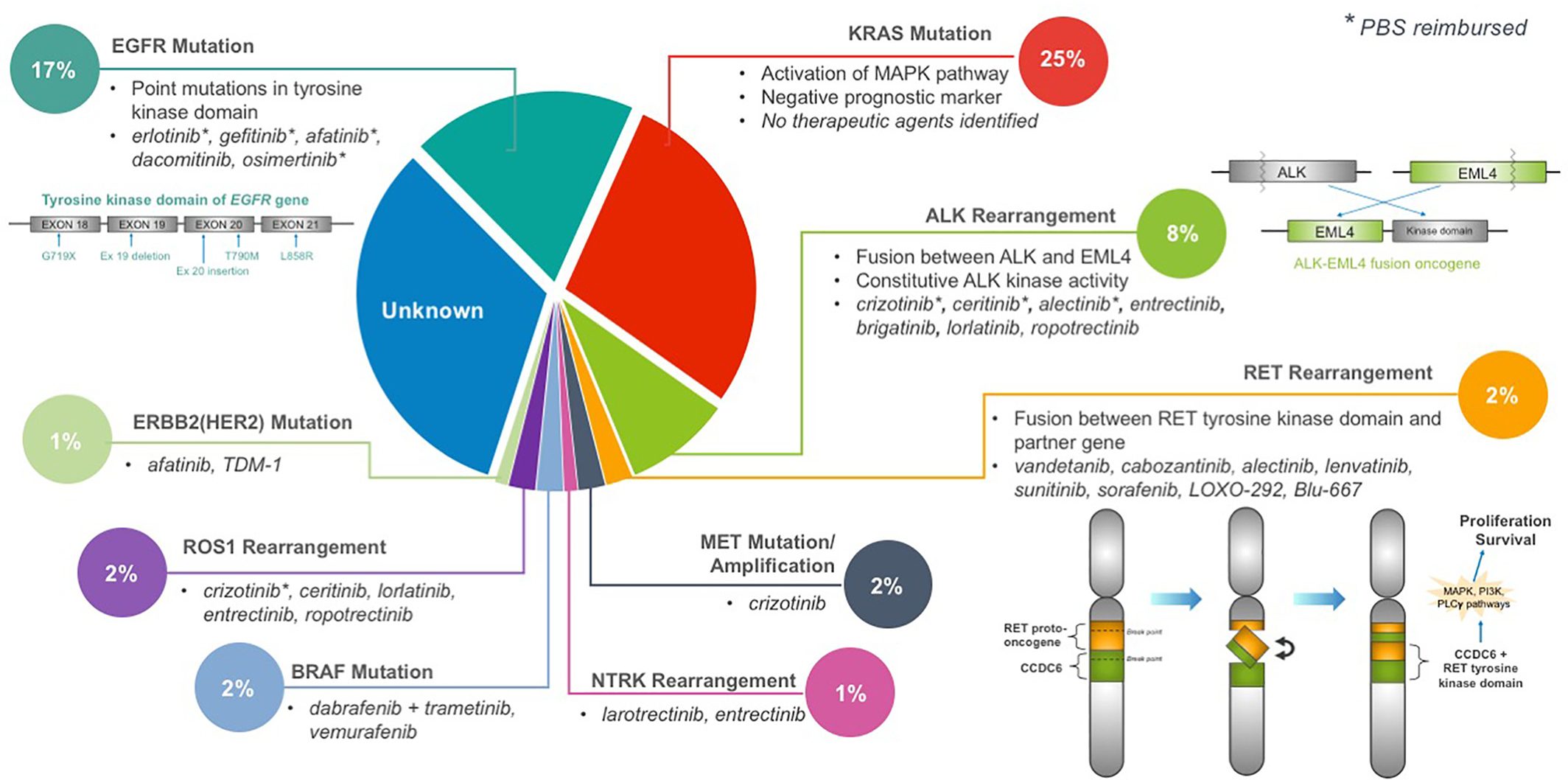
Targeted therapy, such as the use of tyrosine kinase inhibitors (TKI), has revolutionized personalized medicine for advanced NSCLC patients. As mentioned in the treatments section TKI interfere with the cancer’s ability to survive and proliferate through inhibiting signals or pathways that promote cancer (Melosky et al.). Every year, new TKI are developed to better target actionable mutations in NSCLC patients (Leighl). This allows for cancer therapeutics to shift from a “one-size-fits-all” approach to a patient-tailored strategy. Here we will explore the oncogenic driver RET fusion, a relatively newly discovered oncogenic driver suitable for targeted therapy.
RET Fusion
Background
Rearrangement during transfection (RET) is a proto-oncogene that encodes for a tyrosine kinase. Normally, the RET gene is important for renal and nervous system development, however when fused with another gene or mutated, RET will trigger downstream effects that promote uncontrolled cell growth and survival (O’Leary et al.; Russo et al.). Currently, there are over 35 fusion partners for RET, with KIF5B, CCDC6 and NCOA4 being the most common. RET fusions are rare, accounting for only 1-2% of lung cancer cases. Patients with RET fusions are typically younger (<60 years of age), never smokers, and have poorly differentiated tumours with high metastatic potential (Subbiah et al.).
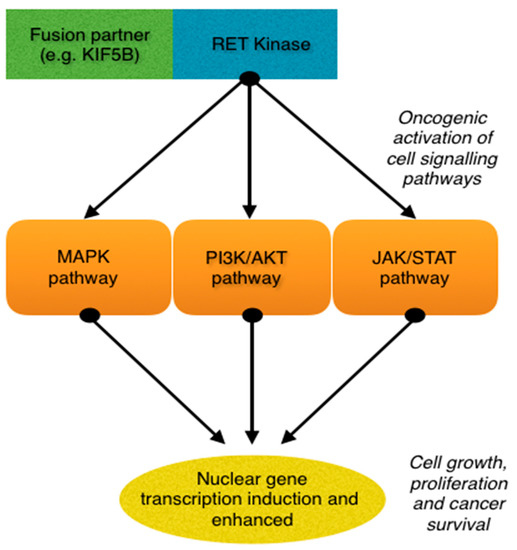
Treatments
Currently, there are no approved agents for RET fusion lung cancers in Canada, therefore patients would likely be treated with chemotherapy or immunotherapy. Unfortunately, the overall survival and progression free survival for these 2 treatments are suboptimal for RET fusion patients (Offin et al.; Shen et al.). Clinical trials for multikinase inhibitors (MKI) have also displayed some promising results, however, overall response rates and progression-free survival are still low compared to the well-known TKI for EGFR, ALK, and ROS1 (Drilon, Hu, et al.). Additionally, since MKI target multiple pathways in order to inhibit RET, these MKI are often associated with high grade adverse events (Ferrara et al.). Recently, new selective RET inhibitors, such as Selpercatinib in the phase I/II study LIBRETTO-001 and Pralsetinib in the phase I/II ARROW study have demonstrated impressive interim results, where overall response rates and progression-free survival are around the range of well-known TKI oncogenic drivers (Drilon, Oxnard, et al.; Gainor et al.). Along with lower grades of adverse events, these 2 drugs are extremely promising for this cohort of patients.
Future Directions
The next step for RET fusion research is to test the effectiveness of MKI or selective RET inhibitors on specific types of RET fusions. In Yoh and colleagues’ phase II study on the MKI drug vandetanib, they saw better response and progression-free survival to vandetanib in patients with CCDC6-RET, compared to KIF5B-RET fusions (Yoh et al.). Though the sample sizes were small, this reveals that some fusion partners may respond better to vandetanib than others. Further research in this direction will allow clinicians to prescribe the most suitable therapy for the patient’s specific cancer. Another next step would be to use next generation sequencing (NGS) and targeted RNA sequencing in the detection of specific fusion partners. In past trials for RET MKI, Fluorescence in situ hybridization (FISH) and reverse transcription polymerase chain reaction (RT-PCR) were the main screening techniques used (Lee et al.; Platt et al.; Yoh et al.). Though these techniques are highly sensitive and effective at detecting RET rearrangements, FISH has troubles with detecting RET fusion partners, while RT-PCR can only detect known fusion partners (Ferrara et al.). Thus, using NGS and targeted RNA sequencing may improve detection, and therefore the targeting, of specific RET fusions.
Case Study
(Case study from Loh et al.; Graphics in the case study were created using Canva; enter full screen mode for the best experience)
Liquid Biopsy in NSCLC

Liquid biopsy is one of the “holy grails” of medical oncology and allows for the analysis of circulating tumour DNA (ctDNA) shed from the primary tumour (Johann et al.). It can be done non-invasively through routine blood sampling, sputum collections, urine tests, or the collection of any liquid (Leighl). Currently surgical biopsies are considered the standard for NSCLC diagnosis but liquid biopsies may be used to overcome drawbacks of surgical biopsies, such as limited tumour tissue access, lack of temporal heterogeneity in the tumour, and cost (Mathai et al.). Liquid biopsies also provide versatile clinical utility as it can be used to detect early stages of cancer, identify genomic abnormalities that could be targeted by TKI, assess the effectiveness of certain therapies, and quantify resistance to therapies (Johann et al.). Through a technique as simple as a blood draw, liquid biopsy enables a quicker and cheaper method for genomic profiling with similar accuracies as tissue biopsies (Pritchett et al.).
Though liquid biopsies have a great potential as a diagnostic tool, one of the major limitations is the lack of standardization, especially for the selection of tumour markers being analyzed (Crowley et al.). A next step for liquid biopsies would be to conduct randomized clinical trials to compare liquid biopsy-guided decisions with tissue-biopsy decisions. Since concordance between the two techniques have been reported high, this next step will allow clinicians to understand whether liquid biopsies are reliable when used alone (Pritchett et al.). If so, this will help speed up the process for identifying patients who will benefit from targeted therapy, therefore allowing them to start targeted treatments immediately, rather than receiving chemotherapy treatments in the interim.
References
Crowley, Emily, et al. “Liquid Biopsy: Monitoring Cancer-Genetics in the Blood.” Nature Reviews. Clinical Oncology, vol. 10, no. 8, Aug. 2013, pp. 472–84. PubMed, doi:10.1038/nrclinonc.2013.110.
Drilon, Alexander, Geoffrey R. Oxnard, et al. “Efficacy of Selpercatinib in RET Fusion-Positive Non-Small-Cell Lung Cancer.” The New England Journal of Medicine, vol. 383, no. 9, 27 2020, pp. 813–24. PubMed, doi:10.1056/NEJMoa2005653.
Drilon, Alexander, Zishuo I. Hu, et al. “Targeting RET-Driven Cancers: Lessons from Evolving Preclinical and Clinical Landscapes.” Nature Reviews Clinical Oncology, vol. 15, no. 3, Mar. 2018, pp. 151–67. DOI.org (Crossref), doi:10.1038/nrclinonc.2017.175.
Ferrara, Roberto, et al. “Clinical and Translational Implications of RET Rearrangements in Non-Small Cell Lung Cancer.” Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer, vol. 13, no. 1, 2018, pp. 27–45. PubMed, doi:10.1016/j.jtho.2017.10.021.
Gainor, Justin F., et al. “Clinical Activity and Tolerability of BLU-667, a Highly Potent and Selective RET Inhibitor, in Patients (Pts) with Advanced RET-Fusion+ Non-Small Cell Lung Cancer (NSCLC).” Journal of Clinical Oncology, vol. 37, no. 15_suppl, American Society of Clinical Oncology, May 2019, pp. 9008–9008. ascopubs.org (Atypon), doi:10.1200/JCO.2019.37.15_suppl.9008.
Johann, Donald J., et al. “Liquid Biopsy and Its Role in an Advanced Clinical Trial for Lung Cancer.” Experimental Biology and Medicine (Maywood, N.J.), vol. 243, no. 3, 2018, pp. 262–71. PubMed, doi:10.1177/1535370217750087.
Lee, S. H., et al. “Vandetanib in Pretreated Patients with Advanced Non-Small Cell Lung Cancer-Harboring RET Rearrangement: A Phase II Clinical Trial.” Annals of Oncology, vol. 28, no. 2, Feb. 2017, pp. 292–97. ScienceDirect, doi:10.1093/annonc/mdw559.
Leighl, Natasha. Expert’s Corner: Lung Cancer. Interview by Janice Li, 5 Dec. 2020, https://play.library.utoronto.ca/play/4a3ae2fc17a78d551d4cd9404e52bc66.
Liquid Biopsy. https://www.massivegenomics.co.ke/pancancer.html. Accessed 5 Dec. 2020.
Loh, Zoe, et al. “RET-Rearranged Non-Small-Cell Lung Cancer and Therapeutic Implications.” Internal Medicine Journal, vol. 49, no. 12, 2019, pp. 1541–45. Wiley Online Library, doi:https://doi.org/10.1111/imj.14654.
Mathai, Roshni Ann, et al. “Potential Utility of Liquid Biopsy as a Diagnostic and Prognostic Tool for the Assessment of Solid Tumors: Implications in the Precision Oncology.” Journal of Clinical Medicine, vol. 8, no. 3, Mar. 2019. PubMed, doi:10.3390/jcm8030373.
Offin, Michael, et al. “Immunophenotype and Response to Immunotherapy of RET-Rearranged Lung Cancers.” JCO Precision Oncology, no. 3, American Society of Clinical Oncology, May 2019, pp. 1–8. ascopubs.org (Atypon), doi:10.1200/PO.18.00386.
O’Leary, Connor, et al. “Rearranged During Transfection Fusions in Non-Small Cell Lung Cancer.” Cancers, vol. 11, no. 5, 5, Multidisciplinary Digital Publishing Institute, May 2019, p. 620. www.mdpi.com, doi:10.3390/cancers11050620.
Platt, Adam, et al. “A Retrospective Analysis of RET Translocation, Gene Copy Number Gain and Expression in NSCLC Patients Treated with Vandetanib in Four Randomized Phase III Studies.” BMC Cancer, vol. 15, Mar. 2015, p. 171. PubMed, doi:10.1186/s12885-015-1146-8.
Pritchett, Michael A., et al. “Prospective Clinical Validation of the InVisionFirst-Lung Circulating Tumor DNA Assay for Molecular Profiling of Patients With Advanced Nonsquamous Non-Small-Cell Lung Cancer.” JCO Precision Oncology, vol. 3, 2019. PubMed, doi:10.1200/PO.18.00299.
Russo, Alessandro, et al. “New Targets in Lung Cancer (Excluding EGFR, ALK, ROS1).” Current Oncology Reports, vol. 22, no. 5, Apr. 2020, p. 48. PubMed, doi:10.1007/s11912-020-00909-8.
Shen, Tianle, et al. “Association Between RET Fusions and Efficacy of Pemetrexed-Based Chemotherapy for Patients With Advanced NSCLC in China: A Multicenter Retrospective Study.” Clinical Lung Cancer, vol. 21, no. 5, Sept. 2020, pp. e349–54. ScienceDirect, doi:10.1016/j.cllc.2020.02.006.
Subbiah, Vivek, et al. “State-of-the-Art Strategies for Targeting RET-Dependent Cancers.” Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology, vol. 38, no. 11, Apr. 2020, pp. 1209–21. PubMed, doi:10.1200/JCO.19.02551.
Yoh, Kiyotaka, et al. “Vandetanib in Patients with Previously Treated RET-Rearranged Advanced Non-Small-Cell Lung Cancer (LURET): An Open-Label, Multicentre Phase 2 Trial.” The Lancet. Respiratory Medicine, vol. 5, no. 1, 2017, pp. 42–50. PubMed, doi:10.1016/S2213-2600(16)30322-8.
