55 5.1: Current implications of checkpoint blockade therapies
Importance of utilizing of checkpoint blockades
Previously in this chapter, we introduced that checkpoint blockade therapies have beneficially promoted T cells to initiate tumour cell death within cancer immunotherapy treatments. Overall, immunotherapies present a great potential in destroying tumours because they alter the body’s immune response by utilizing their own immune checkpoint proteins (Li et al. 31). These checkpoint proteins are essential and have evolved to prevent strong immune responses, which would normally result in normal tissues becoming damaged when coming in contact with foreign antigens (Li et al. 31). Tumours can then take advantage of this and the associated circuits of these checkpoint proteins that normally function to regulate self-tolerance within the immune system. Immune checkpoint blockades (ICBs) are therefore used as inhibitors that prevent the limitation of the T cell response, and therefore promote antitumor activity instead (Li et al. 31). Through their implementation within immunotherapy, ICBs have provided a novel way to target cancers that normally thrive through the avoidance of the immune system.
Successes of checkpoint blockade therapy
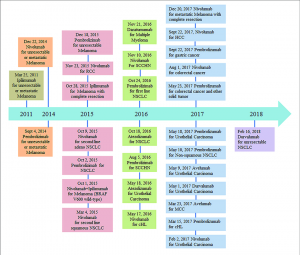
Two of the most commonly used and well-known immune checkpoints include cytotoxic T-lymphocyte protein 4 (CTLA-4) along with programmed cell death protein 1 pathway (PD1/PDL1) (Song et al. 157-172). CTLA-4 has been notably used towards the regulation of early T-cell proliferation within lymph nodes and the suppression of dendritic cells using Treg cells (Buchbinder and Desai 98-106). In comparison, and much later within the immune response, PD1 acts to inhibit effector T cells and NK cells within peripheral tissues and induce Treg cell differentiation (Buchbinder and Desai 98-106). The introduction of antibodies used to provide these blockades began in 2011 where ipilimumab, an anti-CTLA-4 antibody, was approved for use in patients with melanoma (Robert 3801). Subsequently, pembrolizumab and nivolumab were anti-PD1 monoclonal antibodies authorized in 2014, and atezolizumab (introduced within 2016) and durvalumab (introduced within 2017) were used as anti-PDL1 antibodies (Robert 3801). A timeline of other checkpoint inhibitors can be found in figure 1. Since these immune checkpoint inhibitors (ICIs) can pair along with other current cancer management treatments, they have been highly successful in targeting aggressive metastatic cancers. As a result, ICIs have been implemented as a more holistic approach taking into account the overall view of the cancer immune environment creating a bridge between oncologists and immunologists (Robert 3801).
Newer studies have been testing combination therapy using both anti-CTLA-4 and anti-PD-1/PDL1 in hopes of possible synergistic effects. Patients have previously shown to have low response rates when using only monotherapies. This was evidently seen within metastatic melanoma patients who had less than 50% of their patients respond effectively to their monotherapy treatments (Rotte). Combination therapies have shown to increase response rates along with median survival times (Rotte). Within non-small-cell lung cancer and associated high tumour mutational burden patients, Hellmann et al. have even shown that progression-free survival using nivolumab (anti-PD1antibody) and ipilimumab (anti-CTLA-4 monoclonal antibody) was significantly longer than that from chemotherapy or nivolumab monotherapy alone (2093-2104). The combination of nivolumab and ipilimumab have also shown promising results within metastatic melanoma, mesothelioma, colorectal cancer with MMR/MSI-H aberrations, advanced renal cell carcinoma, and sarcoma (Rotte).
Current challenges with combination therapy
The first challenge is testing for other combinations of PD1 and CTLA-4 blockers. There have been successful findings using nivolumab and ipilimumab, however, the effects of other blockade combinations are uncertain. Future studies may benefit analyzing whether other combinations may lead to successful results with few adverse effects (Rotte). The second challenge is that to see efficient results, the dosage of each PD1 and CTLA-4 blocker must be considered as it varies per cancer type. Therefore, to limit the severity of side effects and the precipitation of autoimmunity, proper dose regimens and sequence administration should be acknowledged within future studies (Rotte).
Test your knowledge:
