61 6.3 Novel Treatment – PDL1 CAR T Cell Therapy
This study aimed to find an improved PD-1/PD-L1 therapy for non-small cell lung carcinoma (NSCLC) tumours for patients (Liu et al.). The primary goal was to find a therapy for epidermal growth factor receptor (EGFR) mutant NSCLC tumours.
Generation Of NSCLC Mouse Models
For experiments using cell lines, A549 and H1299 cells were used as the EGFR-WT NSCLC cell lines. Additionally, HCC827, PC19, and H1975 cells were used as the EGFR mutant NSCLC cell lines. BEAS-2B cells were used as a control lung cell model. For experiments using mouse models, NSG mice were used because they are immunodeficient and are commonly used for cancer xenograft modelling. Mice were subcutaneously inoculated with one of the cell lines mentioned previously to induce either EGFR-WT or EGFR mutant tumour growth; they were the NSCLC models. Then, CAR constructs were created: a PD-L1 CAR construct was used for the treatment group and a CD19 CAR construct was made for the control group. Each construct was then transduced into separate groups of T cells and the T cells were injected into the tail of the NSG NSCLC containing mice. These PD-L1 CAR T cells were used as the therapy.

The first thing that was discovered was that the EGFR mutant cell lines used also contained a high level of PD-L1 expression (PD-L1high). Conversely, the EGFR WT cell lines that were used contained low levels of PD-L1 expression (PD-L1low).
Treatment for PD-L1high tumours
When PD-L1 blockade CAR T cell therapy was used on the HCC827, PC19, and H1975 cell lines, there was a significant cytotoxic effect; in other words, CAR T cell therapy results in cell lysis in NSCLC tumours that express high levels of PD-L1. Additionally, as seen in Figure 2, when PD-L1 CAR T cell therapy was used in the mouse models containing HCC827 tumours there was a drastic reduction in tumour size and complete elimination of tumours in most mice. Then, to see if the effects of CAR T cell therapy were long-lasting and effective against recurring tumours, HCC827 cells were reinjected into the same mice. As seen in the figure, by day 84, the tumours were almost completely eliminated again, demonstrating the sustained antitumor effects of PD-L1 CAR T cell therapy on this cell line. To validate these findings, the experiment was repeated in H1975 tumours and it was shown that the size is also significantly reduced in response to CAR T cell therapy. There was also a drastic reduction in PD-L1 expression after CAR T cell therapy was carried out on the tumours, suggesting that the therapy acts by directly targeting and killing PD-L1 positive cancer cells. Importantly, other tissues were analyzed after PD-L1 CAR T cell therapy was performed on the mice to see if the treatment has any adverse side effects on other crucial organs. It was found that the spleen and lung morphology was not altered in response to CAR T cell therapy, which suggests that the treatment has a directed effect with no off-target effects. Because PD-L1 CAR T cell therapy is effective on numerous EGFR mutant tumours, has a targeted and specific effect towards PD-L1 positive NSCLC cells, and appears to have no adverse off-site effects, this could be a promising therapy to move into human trials.

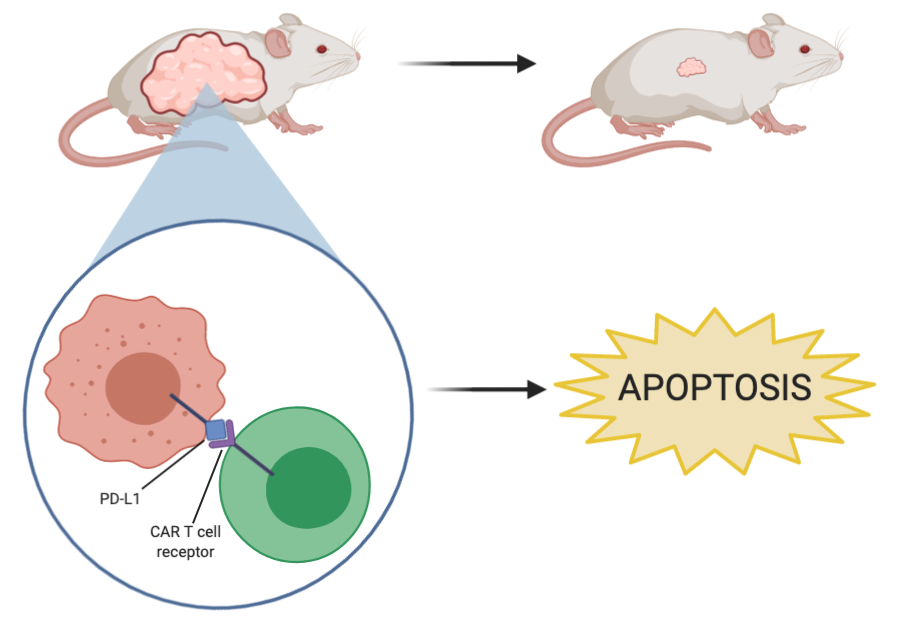
Treatment for PD-L1low tumours
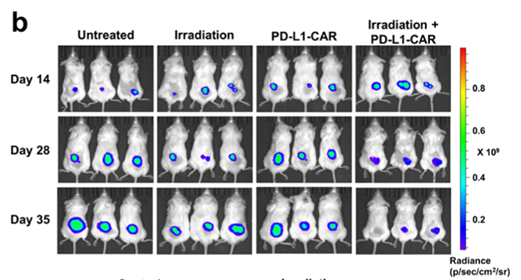
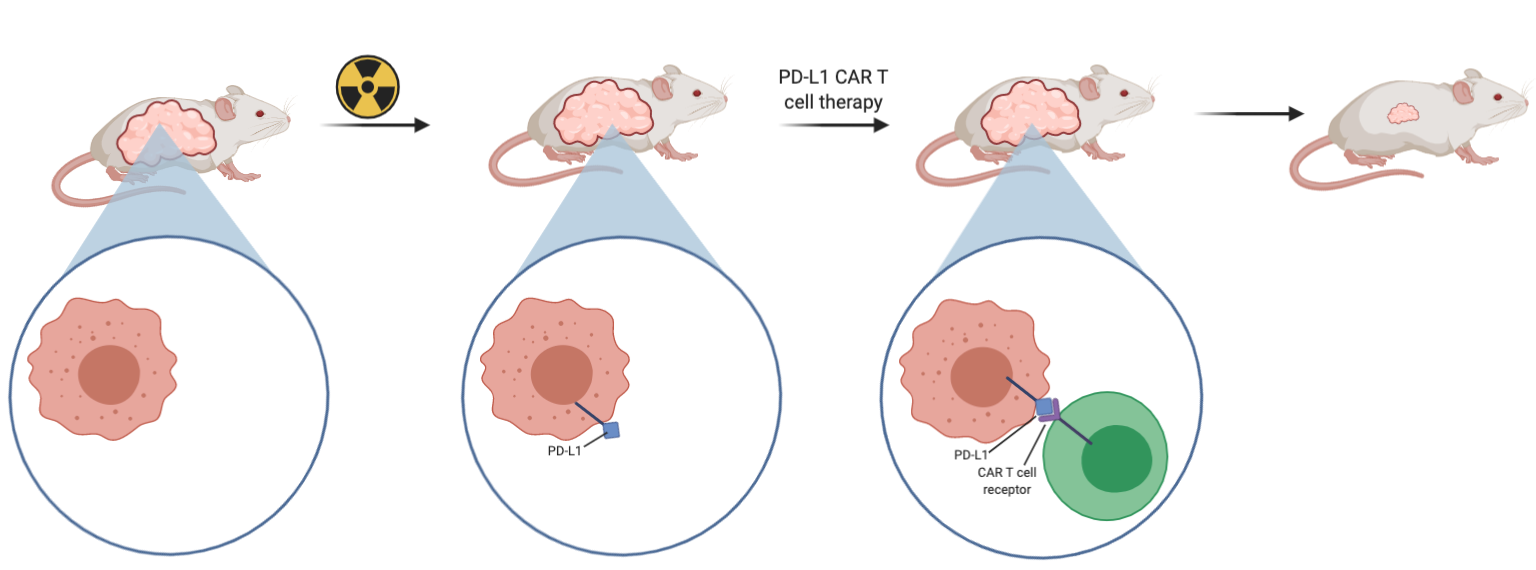
When PD-L1 CAR T cell treatment was used on the A549 and H1299 cells, there was no cytotoxic effect and cell lysis seen, suggesting that the therapy is not effective against tumours that express low levels of PD-L1. In an attempt to solve this problem, they wanted to increase the expression of PD-L1 in the NSCLC using radiation, so that the application of the therapy would be effective. Using the NSG mouse models with A549 tumours inoculated in them, it was found that combination therapy of radiation followed by PD-L1 CAR T cell therapy was effective at reducing tumour growth (Figure 4). As seen in Figure 4, radiation by itself was not enough to halt growth or reduce tumour size. This adds further evidence that the CAR T cell therapy is exerting its effects by directly targeting the PD-L1 positive cells.
References
Liu, Ming, et al. “Targeting PD-L1 in Non-Small Cell Lung Cancer Using CAR T Cells.” Oncogenesis, vol. 9, no. 8, 8, Nature Publishing Group, Aug. 2020, pp. 1–11. www.nature.com, doi:10.1038/s41389-020-00257-z.
There was particular interest in EGFR mutations in NSCLC because tumours containing these mutations do not respond well to PD-1/PD-L1 blockade therapy. Therefore, another treatment must be found for this group.
