27 3.6 Bioengineering at the N-Terminus of Melittin
Overview
Now that the anticancer properties of melittin have been studied, it is also critical to understand the affinity of melittin for breast cancer cells. Previous research has explored how blocking integrin-mediated interactions between TNBC cell membranes and the extracellular matrix has the ability to decrease cancer progression (Li et al., 2016). This section explores how the N-terminal amino acid sequence of melittin may be altered such that its affinity for integrins in breast cancer cell membranes is enhanced.
Breast Cancer Cell Targeting: Enhanced Ability of RGD1-Melittin
Concept and Methodology Review
TGF-β3 is an isoform of transforming growth factor beta; this isoform is known to inhibit the progression of breast cancer.
Watch this video to learn more about the role of integrins in cancer metastasis:
By way of bioengineering technology, researchers developed a novel RGD1-melittin peptide. They chose this RGD motif as it is similar to a sequence expressed by TGF-β3, which is a cytokine that plays a role in halting breast cancer progression (Dong et al., 2007). This RGD motif has been shown to interact with various integrin isoforms on the plasma membranes of breast cancer cells.
When both normal melittin and RGD1-melittin were tested on T11 cells, the IC₅₀ was relatively similar for both peptides, indicating that they both exhibit the same level of anticancer activity. However, the amount of time it took for RGD1-melittin to exert these anticancer effects on TNBC cells was much shorter than for normal melittin. Researchers determined this by treating SUM159 and HDFa cells with the IC₅₀ of both melittin and RGD1-melittin for a 24-hour window, and then comparing the percentage of viable cells immediately afterwards. This tells us that RGD1-melittin has a much higher affinity for TNBC cells than melittin, due to the enhanced targeting of integrins in the plasma membrane.
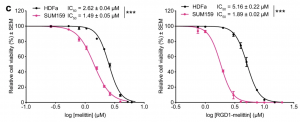
Analyzing the therapeutic window of melittin and RGD1-melittin on TNBC cells (SUM159) as compared to healthy cells (HDFa) (Duffy et al., 2020).
Key Takeaways
- Adding an RGD motif at the N-terminus of melittin enhances its ability to target TNBC cell membranes.
- RGD1-melittin requires a shorter amount of time than normal melittin to exhibit anticancer effects on TNBC cells.
- RGD1-melittin is no more potent than normal melittin in the way of anticancer activity.
- Disrupting integrins in the plasma membrane of breast cancer cells disrupts breast cancer progression.
References
- Dong M., How T., Kirkbride K. C., Gordon K. J., Lee J. D., Hempel N., Kelly P., Moeller B. J., Marks J. R., & Blobe G. C. (2007). The type III TGF-β receptor suppresses breast cancer progression. The Journal of Clinical Investigation, 117(1), 206-217. doi:10.1172/JCI29293
- Li W., Liu C., Zhao C., Zhai L., & Lv S. (2016). Downregulation of β3 integrin by miR-30a-5p modulates cell adhesion and invasion by interrupting Erk/Ets‑1 network in triple-negative breast cancer. International Journal of Oncology, 48(3), 1155-1164. doi: 10.3892/ijo.2016.3319
